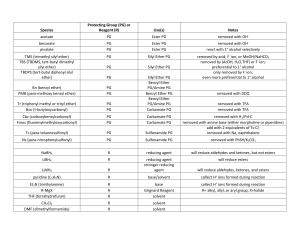
reduction c=o worksheet
advertisement

Princess Norah Bint Abdulrahman University Collage of pharmacy Pharmaceutical Chemistry Department PHS243 Organic Chemistry I Reduction of carbonyl compounds Lab worksheet Reduction of benzaldehyde 1- dissolve 13.5g of KOH in 13ml of H2O then add it in ice bath. 2-add the solution in glass bottle and add 15ml of benzaldehyde, close the bottle and shake will, then leave it for 24h. 3-after 24h, add 50ml of H2O and shake until the solution dissolve. 4- transfer the solution to separating funnel, wash the remain with 15ml of ether and add it to the solution. 5-shake the solution in the separating funnel to separate benzyl alcohol, collect the ether layer (the upper layer), and repeat the process twice more (each with 12ml ether). (keep both the ether and the aqueous layer) 6-pass the collected solution (ether layer) in to ordinary funnel lined with filter paper and saturated sodium sulphate powder. 7- add 5ml of 10% sodium carbonate , and separate it , then wash it with 5ml H2O . 8-leave to dry, then collect the powder and calculate the % of benzyl alcohol. 9- to the aqueous layer add 40ml conc. HCl , 40ml of H2O and 50g of ice. 10-filter the benzoic acid by vacum filtration. 11-wight the powder and calculate the % of benzoic acid.






![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)