Private room, except when directed otherwise by Infection Control.
advertisement

Section: G
Policy Number: 10
Section Title:
Health Center Clinical
Scope:
Clinical Protocols
Policy Title:
Infection Control and Prevention of
Transmission Policy
Date Approved by Board of Directors:
xx/xx/xxxx
1. Summary
Within the health-care setting, general infection control procedures have been developed to
minimize the risk of patient acquisition of infection from contact with contaminated devices,
objects, or surfaces &/or of transmission of an infectious agent from health-care workers to
patients. Such procedures also protect workers from the risk of becoming infected.
General infection-control procedures are designed to prevent transmission of a wide range of
microbiological agents and to provide a wide margin of safety in the varied situations
encountered in the health-care environment.
La Comunidad Hispana (LCH) will implement this infection control policy across the environment
of care as a general practice, and including specific controls wherever and whenever healthcare
workers and/or clients are deemed to be at risk.
2. Key Standards
2.1 Universal precautions
Universal Precautions (UP) are intended to prevent parenteral, mucous membrane, and nonintact
skin exposures of health-care workers to bloodborne pathogens. In addition to emphasizing
prevention of needlestick injuries and the use of traditional barriers such as gloves and gowns, UP
expanded Blood and Body Fluid Precautions to include use of masks and eye coverings to prevent
mucous membrane exposures during certain procedures and the use of individual ventilation
devices when the need for resuscitation was predictable.
2.1.1 Body fluids to which universal precautions apply
Universal precautions apply to blood and to other body fluids containing visible blood.
Occupational transmission of HIV and HBV to health-care workers by blood is documented.
Universal precautions also apply to semen and vaginal secretions. Although both of these fluids
have been implicated in the sexual transmission of HIV and HBV, they have not been implicated in
occupational transmission from patient to health-care worker.
1
2.1.2 Body fluids to which universal precautions do not apply:
Precautions do not apply to feces, nasal secretions, sputum, sweat, tears, urine, and vomitus
unless they contain visible blood. The risk of transmission of HIV and HBV from these fluids and
materials is extremely low or nonexistent. Human breast milk has been implicated in perinatal
transmission of HIV, and HBsAg has been found in the milk of mothers infected with HBV;
however, occupational exposure to human breast milk has not been implicated in the
transmission of HIV nor HBV infection to health-care workers. Universal precautions do not apply
to saliva. General infection control practices already in existence, including the use of gloves for
digital examination of mucous membranes and hand washing after exposure to saliva, should
further minimize the minute risk, if any, for salivary transmission of HIV and HBV.
2.2 Standard precautions
Standard Precautions synthesize the major features of Universal Precautions (designed to reduce
the risk of transmission of bloodborne pathogens) and Body Substance Isolation (designed to
reduce the risk of transmission of pathogens from moist body substances). Standard Precautions
apply to
a) blood;
b) all body fluids, secretions, and excretions, except sweat, regardless of whether or not
they contain visible blood;
c) nonintact skin;
d) mucous membranes.
Standard precautions are designed to reduce the risk of transmission of microorganisms from
both recognized and unrecognized sources of infection in health care settings.
2.3 Human Immunodeficiency Virus (HIV) and Hepatitis B Virus (HBV) transmission
Although the potential for HBV transmission in the workplace setting is greater than for HIV, the
modes of transmission for these two viruses are similar. Both have been transmitted in
occupational settings only by percutaneous inoculation or contact with an open wound, nonintact
(e.g., chapped, abraded, weeping, or dermatitic) skin, or mucous membranes to blood, bloodcontaminated body fluids, or concentrated virus. Blood is the single most important source of HIV
and HBV in the workplace setting. Protection measures against HIV and HBV for workers should
focus primarily on preventing these types of exposures to blood as well as on delivery of HBV
vaccination.
The risk of infection with HIV following one needle-stick exposure to blood from a patient known
to be infected with HIV is approximately 0.5%. This rate of transmission is considerably lower than
that for HBV, probably as a result of the significantly lower concentrations of virus in the blood of
HIV-infected persons. Though inadequately quantified, the risk from exposure of nonintact skin or
mucous membranes is likely to be far less than that from percutaneous inoculation.
2.4
Transmission-based precautions
Transmission-Based Precautions are designed for patients documented or suspected to be
infected or colonized with highly transmissible or epidemiologically important pathogens for
which additional precautions beyond Standard Precautions are needed to interrupt transmission
2
in hospitals. There are three types of Transmission-Based Precautions: Airborne Precautions,
Droplet Precautions, and Contact Precautions. They may be combined for diseases that have
multiple routes of transmission. When used either singularly or in combination, they are to be
used in addition to Standard Precautions. (See attachment A)
2.4.1 Contact Precautions
Contact, or touch, is the most common and most significant mode of transmission of
infectious agents. Patients in Contact Precautions include those infected or colonized with
Clostridium difficile ("C. diff"), rotavirus, or other organisms. Contact transmission can
occur by directly touching the patient, through contact with the patient’s environment, or
by using contaminated gloves or equipment.
Patients with Contact Precautions require:
• Private Room.
• Dedicated, disposable equipment (e.g., stethoscope, blood pressure cuff,
thermometer, etc.).
• Shared equipment is to be cleaned with hospital disinfectant (e.g. disposable
detergent disinfectant-impregnated wipes) after each use.
• Alert staff to type of precautions being used.
Healthcare workers caring for patients in Contact Precautions must:
• Put on gloves before entering the room
• Put on a disposable gown prior to entering the patient’s room when direct
contact with the patient or the patient’s environment is anticipated.
• Remove and discard gloves and gown and clean hands before leaving the
patient’s room or, in semi-private room or multi-bed bay situation, before leaving
the patient’s immediate vicinity.
2.4.2 Droplet Precautions
Droplets are formed when a person coughs, sneezes, speaks, spits, sings, or undergoes
oral or tracheal/bronchial suctioning. Transmission occurs when droplets containing
microorganisms generated from an infected person are propelled a short distance (about
3 feet), and may come in contact with another person’s conjunctivae or mucous
membranes (eyes, nose or mouth).
Diseases transmitted by the droplet route include influenza, and meningococcal
meningitis. Droplets do not remain suspended in the air, and are not transmitted by the
airborne route. Caregivers must wear a mask that covers the mouth and nose (regular
surgical or paper mask), and eye protection (safety goggles, fluid shield).
Patients in Droplet Precautions require:
• Private room, except when directed otherwise by Infection Control.
• Alert staff to type of precautions being used
Family
A. Family will be educated regarding the transmission of droplet-borne diseases:
• Hand hygiene with alcohol based hand rub or soap and water should be
performed regularly and always upon leaving the patient’s room.
3
• Risk of acquisition of droplet-borne diseases is reduced through the use of
personal protective equipment (i.e., surgical mask with eye shield or goggles).
B. Nursing staff must instruct family/visitors to clean hands after contact with patient
secretions or contact with immediate patient environment.
2.4.3 Airborne Precautions
When a person infected with Tuberculosis, Measles, and Chicken Pox coughs, sneezes,
speaks, spits, sings, or undergoes oral or tracheal/bronchial suctioning, droplet nuclei
(particles sized 5 microns or smaller), which carry the infectious organism may be released
into the air and be carried via air currents.
Patients in Airborne Precautions require:
• Private Negative Pressure Isolation Room (NPIR) in the event of confirmed active
TB. IF no room is available, patient room will be left empty for 1 hour with door
closed after patient is transferred to appropriate facility.
• All persons entering the room of a patient with suspected or confirmed
tuberculosis MUST wear an N-95 respirator mask.
Healthcare workers or family members susceptible to chickenpox or measles MAY NOT
enter the patient’s room; healthcare workers immune to chickenpox or measles may enter
the room without wearing a mask. The varicella vaccination is NOT 100% effective in
conferring immunity to chickenpox. Health care workers who have not had the disease
but have been vaccinated shall refrain from entering the room of a patient with
chickenpox or disseminated zoster when there are other immune caregivers available.
• Confinement to their room except for essential purposes, in which case, a
regular mask (surgical or paper) is worn by the patient at all times outside the
negative pressure environment. (Patients with airborne transmitted diseases are
not required to wear an N95 respirator.)
• Alert staff to type of precautions being used
Family members
A. For patients with suspected or confirmed tuberculosis: Family members will wear a
surgical mask that is secured and snugly fitted.
3. Employer Responsibilities
3.1
General
This policy contains a detailed description of the procedures employees at LCH shall follow in
order to protect themselves, staff, and clients from infection transmission in the workplace. LCH’s
duties as an employer shall also contribute to an environment of safety designed for mitigation of
infectious transmission of disease.
3.2
Classification of work activity.
Employee activities are classified according to the standards set out in the Joint Advisory Work
Classification (Appendix B). LCH employees engaged in Category I activities shall use the
appropriate protective equipment. Personal protective equipment (PPE) shall be available to all
workers when they engage in Category I or II activities.
4
a) LCH employees potentially involved in Category I activities as part of their scope of
practice as defined in their job description include licensed and non-licensed personnel
providing direct patient care. All health center staff may potentially be involved in
Category II activities in the event of an emergency, and will also have PPE available to
them.
b) All LCH employees shall follow protocols and procedures related to their scope of practice
and specific to the type of activity being performed. All employees shall undergo
orientation and training to their job and scope of activities to be performed in their
position. Ongoing training shall be performed and assessed annually.
3.3. Preventing transmission of Hepatitis B Virus to workers
All workers whose jobs involve participation in tasks or activities with exposure to blood or other
body fluids to which universal precautions apply (as defined above) should be vaccinated with
hepatitis B vaccine.
3.5. Workers with immunosuppression
Workers with impaired immune systems are at increased risk of acquiring or experiencing serious
complications of infectious disease. Of particular concern is the risk of severe infection following
exposure to other persons with infectious diseases that are easily transmitted if appropriate
precautions are not taken (e.g., measles, varicella). Any worker with an impaired immune system
should be counseled about the potential risk associated with providing health care to persons
with any transmissible infection and should continue to follow existing recommendations for
infection control to minimize risk of exposure to other infectious agents.
The question of whether workers infected with HIV can adequately and safely be allowed to
perform patient-care duties or whether their work assignments should be changed must be
determined on an individual basis. These decisions should be made by the worker's personal
physician(s) in conjunction with the employer's medical advisors.
4. Use of Protective Barriers and PPE
Universal precautions are intended to supplement rather than replace recommendations for
routine infection control, such as handwashing and using gloves to prevent gross microbial
contamination of hands. All employees protective barriers to prevent exposure to blood, body
fluids containing visible blood, and reduce the risk of exposure of the health-care worker's skin or
mucous membranes to potentially infective materials. The type of protective barrier(s) should be
appropriate for the procedure being performed and the type of exposure anticipated. Because
specifying the types of barriers needed for every possible clinical situation is impractical, some
judgment must be exercised.
Examples of protective barriers include gloves, gowns, masks, and protective eyewear.
4.1
General risk reduction
The risk of nosocomial transmission of HIV, HBV, and other bloodborne pathogens can be
minimized if health-care workers use the following general guidelines:
5
a) Take care to prevent injuries when using needles, scalpels, and other sharp
instruments or devices; when handling sharp instruments after procedures; when
cleaning used instruments; and when disposing of used needles.
b) Do not recap used needles by hand; do not remove used needles from disposable
syringes by hand; and do not bend, break, or otherwise manipulate used needles by
hand.
c) Place used disposable syringes and needles, scalpel blades, and other sharp items in
puncture-resistant containers for disposal.
d) Locate the puncture-resistant containers as close to the use area as is practical.
4.2
Personal protective equipment
Appropriate personal protective equipment should be made available routinely by LCH to reduce
the risk of exposure as defined above. For many situations, the chance that the health center staff
will be exposed to blood and other body fluids to which universal precautions apply can be
determined in advance.
If the chance of being exposed to blood is high, the worker should put on protective attire before
beginning patient care. Appendix C sets forth examples of recommendations for personal
protective equipment in clinical settings; the list is not intended to be all-inclusive.
4.2.1. Gloves
Disposable gloves should be a standard component of clinic equipment, and should be donned by
all personnel prior to initiating any emergency patient care tasks involving exposure to blood or
other body fluids to which universal precautions apply. Gloves should reduce the incidence of
contamination of hands, but they cannot prevent penetrating injuries due to needles or other
sharp instruments. Extra pairs should always be available. Considerations in the choice of
disposable gloves should include dexterity, durability, fit, and the task being performed. Thus,
there is no single type or thickness of glove appropriate for protection in all situations. For
situations where large amounts of blood are likely to be encountered, it is important that gloves
fit tightly at the wrist to prevent blood contamination of hands around the cuff. While wearing
gloves, avoid handling personal items, such as combs and pens, that could become soiled or
contaminated.
Gloves that have become contaminated with blood or other body fluids to which universal
precautions apply should be removed as soon as possible, taking care to avoid skin contact with
the exterior surface. Contaminated gloves should be placed in bags that prevent leakage and
should be disposed of or, in the case of reusable gloves, cleaned and disinfected properly.
While wearing gloves, clinicians should observe “clean technique” standards.
The following general guidelines for glove use are recommended:
a) Use sterile gloves for procedures involving contact with normally sterile areas of the
body.
6
b) Use examination gloves for procedures involving contact with mucous membranes,
unless otherwise indicated, and for other patient care or diagnostic procedures that do
not require the use of sterile gloves.
c) Change gloves between patient contacts.
d) Do not wash or disinfect surgical or examination gloves for reuse. Washing with
surfactants may cause "wicking," i.e., the enhanced penetration of liquids through
undetected holes in the glove. Disinfecting agents may cause deterioration.
e) Use general-purpose utility gloves (e.g., rubber household gloves) for housekeeping
chores involving potential blood contact and for instrument cleaning and
decontamination procedures. Utility gloves may be decontaminated and reused but
should be discarded if they are peeling, cracked, or discolored, or if they have
punctures, tears, or other evidence of deterioration.
4.2.2. Glove use for phlebotomy
Follow the guidelines in section 4.2.1. The likelihood of hand contamination with blood
containing HIV, HBV, or other bloodborne pathogens during phlebotomy depends on
several factors:
a)
the skill and technique of the health-care worker,
b) the frequency with which the health-care worker performs the procedure (other
factors being equal, the cumulative risk of blood exposure is higher for a health-care
worker who performs more procedures),
c)
whether the procedure occurs in a routine or emergency situation (where blood
contact may be more likely),
d) the prevalence of infection with bloodborne pathogens in the patient population.
4.2.3 Gowns, and other protective clothing
As per the Occupational Safety and Health Act, masks, gowns, aprons, lab coats, clinic
jackets or similar outer garments shall be worn and used in accordance with the level of
exposure encountered. Management of the patient who is not bleeding, and who has no
bloody body fluids present, should not routinely require use of barrier precautions.
Protective clothing should be worn to protect clothing from splashes with blood and/or
other body fluids. If large splashes or quantities of blood and/or other body fluids are
present or anticipated, impervious gowns or aprons should be worn.
4.2.4 Masks, eye protection and face shields
Masks and protective eyewear or face shields should reduce the incidence of
contamination of mucous membranes of the mouth, nose, and eyes. As per the
Occupational Safety and Health Act, masks and eyewear (e.g., safety glasses), and/or face
shields should be worn whenever splashes, sprays, or droplets of blood or other infectious
7
material may be generated and eye, nose, or mouth contamination can be reasonably
anticipated.
4.2.5 Resuscitation equipment
No transmission of HBV or HIV infection during mouth-to-mouth resuscitation has been
documented. However, because of the risk of salivary transmission of other infectious
diseases (e.g., herpes simplex and Neisseria meningitidis) and the theoretical risk of HIV
and HBV transmission during artificial ventilation of trauma victims, disposable airway
equipment or resuscitation bags should be used. Disposable resuscitation equipment and
devices should be used once and disposed of or, if reusable, thoroughly cleaned and
disinfected after each use according to the manufacturer's recommendations.
5. Disinfection, Decontamination, and Disposal
The CDC precaution guidelines described below should be routinely followed.
5.1. Needle and sharps disposal
All workers should take precautions to prevent injuries caused by needles, scalpel blades, and
other sharp instruments or devices during procedures; when cleaning used instruments; during
disposal of used needles; and when handling sharp instruments after procedures.
5.1.1 To prevent needle-stick injuries, needles should not be recapped, purposely bent or
broken by hand, removed from disposable syringes, or otherwise manipulated by hand.
5.1.2 After they are used, disposable syringes and needles, scalpel blades, and other sharp
items should be placed in puncture-resistant containers for disposal.
5.1.3 The puncture-resistant containers should be located as close as practical to the use
area.
5.1.4 Reusable needles should be left on the syringe body and should be placed in a
puncture-resistant container for transport to the reprocessing area.
5.2. Hand washing
Hands and other skin surfaces should be washed immediately and thoroughly if contaminated
with blood, other body fluids to which universal precautions apply, or potentially contaminated
articles. Hands should always be washed after gloves are removed, even if the gloves appear to be
intact. Hand washing should be completed using the appropriate facilities, such as utility or
restroom sinks. Waterless antiseptic hand cleanser should be provided to use when hand-washing
facilities are not available. When hand-washing facilities are available, wash hands with warm
water and soap. The manufacturer's recommendations for the product should be followed.
5.3.
Cleaning and decontaminating spills of blood
8
All spills of blood and blood-contaminated fluids should be promptly cleaned up using an EPAapproved germicide or a 1:100 solution of household bleach in the following manner while
wearing gloves. Visible material should first be removed with disposable towels or other
appropriate means that will ensure against direct contact with blood. If splashing is anticipated,
protective eyewear should be worn along with an impervious gown or apron that provides an
effective barrier to splashes. The area should then be decontaminated with an appropriate
germicide. Hands should be washed following removal of gloves. Soiled cleaning equipment
should be cleaned and decontaminated or placed in an appropriate container and disposed of.
5.4. Decontamination and laundering of protective clothing
Protective work clothing contaminated with blood or other body fluids to which universal
precautions apply should be placed and transported in bags or containers that prevent leakage.
Personnel involved in the bagging, transport, and laundering of contaminated clothing should
wear gloves. Protective clothing and work uniforms should be washed and dried according to the
manufacturer's instructions. Boots and leather goods may be brush-scrubbed with soap and hot
water to remove contamination.
6. Hazardous Waste Disposal
LCH contracts for biohazardous waste disposal. The following guidelines shall be followed in
disposal of infectious waste.
6.1
General infectious waste disposal
The relative risk of disease transmission and application of local regulations, which vary widely,
determine the selection of procedures for disposal of infectious waste. In all cases, local
regulations should be consulted prior to developing disposal procedures and followed.
Infectious waste should either be incinerated or should be decontaminated before disposal in a
sanitary landfill. Bulk blood, suctioned fluids, excretions, and secretions may be carefully poured
down a drain connected to a sanitary sewer, where permitted. Sanitary sewers may also be used
to dispose of other infectious wastes capable of being ground and flushed into the sewer, where
permitted. Sharp items should be placed in puncture-proof containers and other bloodcontaminated items should be placed in leak-proof plastic bags for transport to an appropriate
disposal location.
6.2
Infectious waste categories
Clinic wastes can be categorized as infectious or noninfectious.
6.2.1 Infectious wastes include human, animal, or biological wastes and any items that
may be contaminated with pathogens.
6.2.2 Noninfectious wastes include toxic chemicals, cytotoxic drugs, and radioactive,
flammable, and explosive wastes.
6.3
Specific types of infectious waste
Infectious wastes classifications common in health centers:
9
6.4
6.3.1
Cultures and stocks of infectious agents and associated biologicals include
specimen cultures from medical and pathological laboratories, cultures and stocks
of infectious agents from research and industrial laboratories, wastes from the
production of biologicals, discarded live and attenuated vaccines, and culture
dishes and devices used to transfer, inoculate, and mix cultures.
6.3.2
Human blood and blood products include blood as well as serum, plasma, and
other blood products.
6.3.3
Pathological wastes include tissues and body fluids that are removed during
surgical procedures.
6.3.4
Contaminated sharps such as hypodermic needles syringes, Pasteur pipettes,
broken glass, and scalpel blades. These items should be considered infectious
wastes because of the possibility of contamination with blood-borne pathogens.
6.3.5
Miscellaneous wastes that are not designated as infectious should be assumed to
be infectious and should be managed as such to maintain consistent levels of
protection for both the environment and for persons handling these wastes.
Miscellaneous wastes include those from contaminated laboratory wastes, and
contaminated equipment.
6.3.6
Wastes from surgery include soiled dressings, sponges, drapes, lavage tubes,
drainage sets, underpads, and surgical gloves.
6.3.7
Contaminated laboratory wastes include specimen containers, slides and cover
slips, disposable gloves, laboratory coats, and aprons.
6.3.8
Contaminated equipment refers to discarded equipment and parts that are used in
patient care, medical and industrial laboratories, research, and the production and
testing of certain pharmaceuticals.
Treatment and disposal methods
6.4.1 Several methods are used for infectious waste treatment, depending on the type of
waste material. These treatment methods include steam sterilization, incineration,
thermal inactivation, gas/vapor sterilization, chemical disinfection, and sterilization by
irradiation. (See Appendix D). In utilizing any treatment and disposal method, follow the
manufacture instructions for use of equipment.
Important Note: To assure the effectiveness of any sterilization or disinfection process,
equipment and instruments must first be thoroughly cleaned of all visible soil.
7. Guidelines for the Management of Health-Care Worker (HCW) Exposures to HIV and HBV.
10
As covered in the 1998 CDC publication Public Health Service Guidelines for the Management of
Health-Care Worker Exposures to HIV and Recommendations for Postexposure Prophylaxis
MMWR 47,
“health-care organizations must have a system that includes written protocols for prompt
reporting, evaluation, counseling, treatment, and follow-up of occupational exposures that may
place HCWs at risk for acquiring any bloodborne infection, including HIV.” The following section
outlines the content of the required protocol.
7.1
Exposure report for HIV
If an occupational exposure occurs, the circumstances and post exposure management shall be
recorded in the HCW's confidential medical record and on an OSHA 300 form for occupational
exposure. HCWs should be educated to report occupational exposures immediately after they
occur, particularly because post exposure prophlaxis is most likely to be effective if implemented
as soon after the exposure as possible.
Relevant information includes:
a)
date and time of exposure;
b) details of the procedure being performed, including where and how the exposure
occurred, and if the exposure was related to a sharp device, the type of device and how
and when in the course of handling the device the exposure occurred;
c)
details of the exposure, including the type and amount of fluid or material and the
severity of the exposure (e.g., for a percutaneous exposure, depth of injury and
whether fluid was injected; or for a skin or mucous-membrane exposure, the
estimated volume of material and duration of contact and the condition of the skin
{e.g., chapped, abraded, or intact});
d) details about the exposure source (i.e., whether the source material contained HIV or
other bloodborne pathogen{s}), and if the source is an HIV-infected person, the stage of
disease, history of antiretroviral therapy, and viral load, if known; and
e)
7.2
details about counseling, post exposure management, and follow-up.
Exposure management for HIV (See Appendix E)
7.2.1 Treatment of an Exposure Site
Wounds and skin sites that have been in contact with blood or body fluids should be
washed with soap and water; mucous membranes should be flushed with water.
7.2.2 Assessment of Infection Risk
After an occupational exposure, the source-person and the exposed HCW should be
evaluated to determine the need for HIV PEP. Follow-up for hepatitis B virus and hepatitis
C virus infections also should be conducted in accordance with previously published CDC
recommendations.
11
7.2.3 Evaluation of exposure.
The exposure should be evaluated for potential to transmit HIV based on the type of body
substance involved and the route and severity of the exposure. Exposures to blood, fluid
containing visible blood, or other potentially infectious fluid (including semen; vaginal
secretions) or tissue through a percutaneous injury (i.e., needlestick or other penetrating
sharps-related event) or through contact with a mucous membrane are situations that
pose a risk for bloodborne transmission and require further evaluation. (See Table 2)
7.2.4 Evaluation and testing of an exposure source.
The person whose blood or body fluids are the source of an occupational exposure should
be evaluated for HIV infection. Information available in the medical record at the time of
exposure or from the source person may suggest or rule out possible HIV infection. If the
source is known to have HIV infection, available information about this person's stage of
infection (i.e., asymptomatic or AIDS), CD4+ T-cell count, results of viral load testing, and
current and previous antiretroviral therapy, should be gathered for consideration in
choosing an appropriate PEP regimen. If the HIV serostatus of the source person is
unknown, the source person should be informed of the incident and, if consent is
obtained, tested for serologic evidence of HIV infection. If consent cannot be obtained
(e.g., patient refuses), procedures should be followed for testing source persons according
to applicable state and local laws. Confidentiality of the source person should be
maintained at all times.
Exposed HCWs should be evaluated for susceptibility to bloodborne pathogen infections.
Baseline testing (i.e., testing to establish serostatus at the time of exposure) for HIV
antibody should be performed. If the source person is seronegative for HIV, baseline
testing or further follow-up of the HCW normally is not necessary. If the source person has
recently engaged in behaviors that are associated with a risk for HIV transmission, baseline
and follow-up HIV-antibody testing (e.g., 3 and/or 6 months post exposure) of the HCW
should be considered. Serologic testing should be made available to all HCWs who are
concerned that they may have been exposed to HIV.
7.3
HIV post exposure prophylaxis (PEP)
The following recommendations apply to situations where an HCW has had an exposure to a
source person with HIV or where information suggests that there is a likelihood that the source
person is HIV-infected.
7.3.1 Explaining PEP to HCWs
Recommendations for chemoprophylaxis should be explained to HCWs who have
sustained occupational HIV exposures. For exposures for which PEP is considered
appropriate, HCWs should be informed that
a)
knowledge about the efficacy and toxicity of drugs used for PEP are limited;
b) only ZDV has been shown to prevent HIV transmission in humans;
12
c)
there are no data to address whether adding other antiretroviral drugs provides any
additional benefit for PEP, but experts recommend combination drug regimens
because of increased potency and concerns about drug-resistant virus;
d) data regarding toxicity of antiretroviral drugs in persons without HIV infection or in
pregnant women are limited for ZDV and not known regarding other antiretroviral
drugs;
e)
any or all drugs for PEP may be declined by the HCW. HCWs who have HIV
occupational exposures for which PEP is not recommended should be informed that
the potential side effects and toxicity of taking PEP outweigh the negligible
risk of transmission posed by the type of exposure.
f)
In consultation with an expert, (See Section 7.6) drugs for PEP should be customized
by using any available information about the source’s antiretroviral history.
7.3.2 Timing of PEP Initiation
PEP should be initiated as soon as possible.
7.3.3 PEP if Serostatus of Source Person is Unknown
If the source person's HIV serostatus is unknown at the time of exposure (including when
the source is HIV negative but may have had a recent HIV exposure), use of PEP should be
decided on a case-by-case basis, after considering the type of exposure and the clinical
and/or epidemiologic likelihood of HIV infection in the source.
7.3.4 PEP if Exposure Source is Unknown
If the exposure source is unknown, use of PEP should be decided on a case-by-case basis.
Consideration should include the severity of the exposure and the epidemiologic likelihood
that the HCW was exposed to HIV.
7.3.5 PEP for Pregnant HCWs
If the HCW is pregnant, the evaluation of risk and need for PEP should be approached as
with any other HCW who has had an HIV exposure. However, the decision to use any
antiretroviral drug during pregnancy should involve discussion between the woman and
her health-care provider regarding the potential benefits and potential risks to her and her
fetus.
7.4.
Follow-up of HCWs exposed to HIV
7.4.1 Post exposure Testing
HCWs with occupational exposure to HIV should receive follow-up counseling, post
exposure testing, and medical evaluation regardless of whether they receive PEP. HIVantibody testing should be performed for at least 6 months post exposure
7.4.2 Monitoring and Management of PEP Toxicity
If PEP is used, drug-toxicity monitoring should be performed at baseline and again 2 weeks
after starting PEP. Clinical judgement, based on medical conditions that may exist in the
13
HCW and any toxicity associated with drugs included in the PEP regimen, should
determine the scope of testing.
7.4.3 Counseling and Education
Although HIV infection following an occupational exposure occurs infrequently, the
emotional impact of the exposure often is substantial. In addition, HCWs are given
seemingly conflicting information. Although HCWs are told that there is a low risk for HIV
transmission, a 4-week regimen of PEP is recommended and they are asked to commit to
behavioral measures (i.e., sexual abstinence or condom use) to prevent secondary
transmission, all of which influence their lives for several weeks to months. Therefore,
access to persons who are knowledgeable about occupational HIV transmission and who
can deal with the many concerns an HIV exposure may raise for the HCW is an important
element of post exposure management.
HIV-exposed HCWs should be advised to use the following measures to prevent secondary
transmission during the follow-up period, especially during the first 6-12 weeks after the exposure
when most HIV-infected persons are expected to seroconvert:
a) use sexual abstinence or condoms to prevent sexual transmission and to avoid
pregnancy;
b) refrain from donating blood, plasma, organs, tissue, or semen.
c) If the exposed HCW is breastfeeding, she should be counseled about the risk for
HIV transmission through breast milk, and discontinuation of breastfeeding should
be considered, especially following high-risk exposures.
There is no need to modify an HCW's patient-care responsibilities to prevent transmission to
patients based solely on an HIV exposure. If HIV seroconversion is detected, the HCW should be
evaluated according to published recommendations for HIV-infected HCWs.
7.5
Exposure Management for Hepatitis B
Evaluation and testing of an exposure source should follow instructions given under HIV section.
7.5.1 Hepatitis B virus post exposure management
For an exposure to a source individual found to be positive for HBsAg, the worker who has
not previously been given hepatitis B vaccine should receive the vaccine series. A single
dose of hepatitis B immune globulin (HBIG) is also recommended, if this can be given
within 7 days of exposure. For exposures from an HBsAg-positive source to workers who
have previously received vaccine, the exposed worker should be tested for antibody to
hepatitis B surface antigen (anti-HBs), and given one dose of vaccine and one dose of HBIG
if the antibody level in the worker's blood sample is inadequate.
If the source individual is negative for HBsAg and the worker has not been vaccinated, this
opportunity should be taken to provide hepatitis B vaccination. If the source individual
refuses testing or he/she cannot be identified, the unvaccinated worker should receive the
hepatitis B vaccine series. HBIG administration should be considered on an individual basis
14
when the source individual is known or suspected to be at high risk of HBV infection.
Management and treatment, if any, of previously vaccinated workers who receive an
exposure from a source who refuses testing or is not identifiable should be individualized.
7.6
Exposure Management for Hepatitis C
Evaluation and testing of an exposure source should follow instructions given under HIV section.
7.9.1 Hepatitis C virus post exposure management
For the person exposed to an HCV-positive source
a) perform baseline testing for anti-HCV and ALT activity; and perform follow-up testing
(e.g., at 4– 6 months) for anti-HCV and ALT activity (if earlier diagnosis of HCV infection is
desired, testing for HCV RNA may be performed at 4 – 6 weeks).
b) Confirm all anti-HCV results reported positive by enzyme immunoassay using
supplemental anti-HCV testing (e.g., recombinant immunoblot assay [RIBA])
Health-care professionals who provide care to persons exposed to HCV in the occupational setting
should be knowledgeable regarding the risk for HCV infection and appropriate counseling, testing,
and medical follow-up. Immunoglobulin and antiviral agents are not recommended for PEP after
exposure to HCV-positive blood.
15
Appendix A.
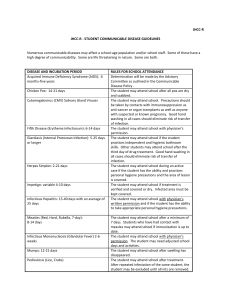
Type and Duration of Precautions Needed for Selected Infections and Conditions
Adapted from “Guidelines for Isolation Precautions in Hospitals” by the CDC Hospital Infection
Control Advisory Committee. Published 1/1/96, updated 1/2007.
Precautions Abbreviations:
Type of precautions:
A= Airborne
D= Droplet
C= Contact
S= Standard
When A, C, and D are specified, also use S.
Duration of precautions:
CN= until off antibiotics and culture-negative;
DH= duration of hospitalization;
DI= duration of illness (with wound lesions, DI means until they stop draining);
U= until time specified in hours (hrs) after initiation of effective therapy;
F= see footnote number.
Infection/Condition
Abscess
Draining, minor or limited
Acquired immunodeficiency syndrome
Precaution Type
S
F(1)
S
Candidiasis, all forms including mucocutaneous
S
Chancroid (soft chancre)
S
Chickenpox (varicella; see F(2) for varicella exposure)
Duration of Precaution
A,C
F (2)
16
Chlamydia trachomatis
Conjunctivitis
Genital
Respiratory
S
S
S
Congenital rubella
C
F (3)
Conjunctivitis
S
S
S
Acute bacterial
Chlamydia
Gonococcal
Endometritis
S
Enterobiasis (pinworm disease, oxyuriasis)
S
Enteroviral infections
Adults
S
German measles (rubella)
D
S
Gonorrhea
Granuloma
venereum)
F (4)
inguinale
(donovanosis,
granuloma
Hepatitis, viral
Type A
Type B -- HBsAg positive
Type C and other unspecified non-A, non-B
Type E
Herpes simplex (Herpesvirus hominis)
Mucocutaneous, disseminated or primary, severe
Mucocutaneous, recurrent (skin, oral, genital)
S
S
S
S
S
C
S
DI
A,C
DI, F (4)
S
F (4)
Herpes zoster (varicella-zoster)
Localized in immunocompromised patient,
or disseminated
Localized in normal patient
17
Human immunodeficiency virus (HIV) infection
Impetigo
S
C
U (24 hrs)
Infectious mononucleosis
S
Influenza
D
DI
Lice (pediculosis)
C
U (24 hrs)
Lyme disease
S
Lymphogranuloma venereum
S
Measles (rubeola), all presentations
A
Meningitis
S
Mumps (infectious parotitis)
D
Respiratory infectious disease, acute (if not covered
elsewhere)
Adults
S
Ringworm (dermatophytosis, dermatomycosis, tinea)
DI
F (5)
S
Rubella (German measles)
D
F (6)
Scabies
C
U (24 hrs)
Streptococcal disease (Group A strep.)
Skin, wound, or burn
Major (1)
Minor or limited (2)
C
S
DI
Toxic shock syndrome
S
18
Syphilis
Skin and mucous membrane, including congenital,
Primary,
Secondary
Latent (tertiary) and seropositivity without lesions
S
S
S
Tinea (fungus infection dermatophytosis,
dermatomycosis, ringworm)
S
Toxic shock syndrome (staphylococcal disease)
S
Trichomoniasis
S
Tuberculosis
Extrapulmonary, draining lesion (including
scrofula)
Extrapulmonary, meningitis
Pulmonary, confirmed or suspected or laryngeal
disease
Skin-test positive with no evidence of current
pulmonary Disease
Urinary tract infection (including pyelonephritis)
Varicella (chickenpox)
Viral diseases
Respiratory (if not covered elsewhere)
Adults
S
S
A
F (7)
S
S
A,C
F (5)
S
19
Footnotes:
(1) Dressing covers and contains drainage adequately.
(2) Maintain precautions until all lesions are crusted. The average incubation period for varicella is
10 to 16 days, with a range of 10 to 21 days. After exposure, use varicella zoster immune globin
(VZIG) when appropriate, and discharge susceptible patients if possible. Place exposed susceptible
patients on Airborne Precautions beginning 10 days after exposure and continuing until 21 days
after last exposure (up to 28 days if VZIG has been given). Susceptible persons should not enter
the room of patients on precautions if other immune caregivers are available.
(3) Place infant on precautions during any admission until 1 year of age, unless nasopharyngeal
and urine cultures are negative for virus after age 3 months.
(4) Persons susceptible to varicella are also at risk for developing varicella when exposed to
patients with herpes zoster lesions; therefore, susceptibles should not enter the room if other
immune caregivers are available.
(5) For 9 days after onset of swelling.
(6) Until 7 days after onset of rash.
(7) Discontinue precautions only when TB patient is on effective therapy, is improving clinically,
and has three consecutive negative sputum smears collected on different days, or TB is ruled out.
Also see CDC "Guidelines for Preventing the Transmission of Tuberculosis in Health-Care
Facilities."
1/6/00
20
Appendix B
Task and Implications for Personal Protective Equipment
Joint Advisory
Work Classification Nature of Task / Activity
Personal Protective Equipment Should Be:
Available
Worn
I.
Direct contact with blood or
other body fluids to which
universal precautions apply
Yes
Yes
II.
Activity performed without
blood exposure but
exposure may occur in
emergency
Yes
No
III.
Task/activity does not entail
predictable or
unpredictable
Exposure to blood
No
No
21
Appendix C
Examples of Recommended Personal Protective Equipment for Worker Protection Against HIV and
HBV Transmission (1) in Prehospital (2) Settings
Task or Activity
Disposable Gloves (3)
Protective Gown
Mask/Eyewear
Bleeding control with
Yes
Yes
Yes
with spurting blood
Bleeding control with Yes
No
No
minimal bleeding
Blood drawing
Yes
No
No
Starting an
Yes
No
No
intravenous (IV) line
Handling and cleaning Yes
No unless soiling
No
instruments with is
likely microbial
contamination
Measuring blood
No
No
No
pressure
Measuring
No
No
No
temperature
Giving an injection
No
No
No
(1) The examples provided in this table are based on application of universal precautions. Universal
precautions are intended to supplement rather than replace recommendations for routine
infection control, such as hand washing and using gloves to prevent gross microbial
contamination of hands (e.g., contact with urine or feces).
(2) Defined as setting where delivery of emergency health care takes place away from a hospital or
other health-care facility.
(3) While not clearly necessary to prevent HIV or HBV transmission unless blood is present, gloves
are recommended to prevent transmission of other agents (e.g., Herpes simplex).
22
Appendix D
Reprocessing Methods for Equipment Used in the Prehospital1 Health-Care Setting (1)
Reprocessing Methods
Destroys
Methods
Use
Sterilization
All forms of microbial life
Steam under pressure
For those instruments or
including high numbers of (autoclave),gas (ethylene devices that penetrate skin or
bacterial spores.
oxide), dry heat, or
contact normally sterile areas
immersion in EPAof the body, e.g., scalpels,
approved chemical
needles, etc. Disposable
sterilant" for prolonged invasive equipment eliminates
period of time, e.g., 6- the need to reprocess these
10 hours or according to
types of items. When
manufacturers'
indicated, however,
instructions. Note: liquid arrangements should be made
chemical
with a health-care facility for
"sterilants"should be
reprocessing of
used only on those
reusable invasive instruments.
instruments that are
impossible to
Sterilize or disinfect with
heat.
High-Level
All forms of microbial life
Hot water pasteurization
For reusable instruments or
Disinfection
except high numbers of
(80-100 C, 30 minutes) or devices that come into contact
bacterial spores.
exposure to an EPAwith mucous membranes (e.g.,
registered "sterilant"
laryngoscope blades,
chemical as above,
endotracheal tubes, etc.).
except for a short
exposure time (10 - 45
minutes or as directed by
the manufacturer).
Intermediate- Mycobacterium tuberculosis, EPA-registered "hospital
For those surfaces that come
Level
Vegetative bacteria, most
disinfectant" chemical
into contact only with intact
Disinfection
viruses, and most fungi, but
germicides that have a
skin, e.g.-, stethoscopes,
does not kill bacterial
label claim for
blood pressure cuffs, splints,
spores.
tuberculocidal activity;
etc., and have been visibly
commercially available
contaminated with blood or
hard-surface germicides
bloody body fluids. Surfaces
or solutions containing
must be precleaned of visible
at least 500 PPM free
material before the germicidal
available chlorine (a
chemical is applied for
1:100 dilution of
disinfection.
common household
bleach - approximately
¼ cup bleach per gallon
of tap water).
23
Low-Level
Disinfection
Environmental
Disinfection
Most bacteria, some viruses,
some
fungi,
but
not
Mycobacterium tuberculosis
or bacterial spores.
EPA-registered "hospital
disinfectants" (no label
claim for tuberculocidal
activity).
These agents are excellent
cleaners and can be used for
routine housekeeping or
removal of soiling in the
absence of visible blood
contamination.
Surfaces include floors,
woodwork, ambulance seats,
countertops, etc.
Environmental surfaces
which have become
soiled, should be cleaned
and disinfected using any
cleaner or disinfectant
agent which is intended
for environmental use.
(1) Defined as setting where delivery of emergency health-care takes place prior to arrival at
hospital or other health-care facility.
IMPORTANT:
To assure the effectiveness of any sterilization or disinfection process, equipment and
instruments must first be thoroughly cleaned of all visible soil.
24
Appendix E
Basic and expanded post exposure prophylaxis regimens
Regimen category
Basic
Expanded
Application
Drug regimen
Occupational HIV exposures for 4 weeks (28 days) of both zidovudine
which there is a recognized 600 mg every day in divided doses (i.e.
transmission risk (Figure 1).
300 mg twice a day, 200 mg three
times a day, or 100 mg every 4 hours)
and lamivudine 150 mg twice a day.
Occupational HIV exposures that Basic regimen plus either indinavir 800
pose an increased risk for
mg every 8 hours or nelfinavir 750 mg
transmission (e.g. larger volume larger volume of blood three times a
of blood and/or higher virus titer day.*
in blood)
* Idinavir should be taken on an empty stomach (i.e. without food or with a light meal)
and with increased fluid consumption (i.e. drinking six 8oz glasses of water throughout
the day); nelfinavir should be taken with meals.
From 2001 CDC: Public Health Service Guidelines for the Management of Health-Care
Worker Exposures to HIV and Recommendations for Postexposure Prophylaxis MMWR.
25
Appendix F
HIV post exposure prophylaxis resources and registries
Resource or registry
National Clinicians' Post exposure Hotline
Antiretroviral Pregnancy Registry
Contact Information
Telephone: (888) 448-4911
or
(888) PEP4HIV
Write: 1410 Commonwealth Drive
Suite 215
Wilmington, NC 28405
Telephone: (800) 258-4263
Fax:
(800) 800-1052
Write: 1410 Commonwealth Drive
Suite 215
Wilmington, NC 28405
Food and Drug Administration (for reporting Telephone: (800) 322-1088
unusual or severe toxicity to anti- retroviral
agents)
CDC (for reporting HIV seroconversions in Telephone: (404) 639-6425
health-care workers who received PEP)
26