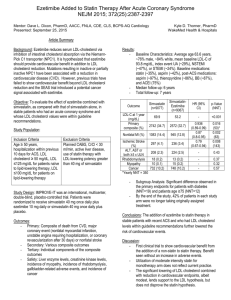
(Rs)
advertisement

Development and Validation of UV Spectrophotometric Area under Curve Method for Estimation of Ezetimibe in Bulk and Tablet Dosage Form K. V. TALWADEKAR1 AND H. K. JAIN1* 1 Department of Quality Assurance Techniques, STES’s Sinhgad College of Pharmacy, Vadgaon (Bk), Off. Sinhgad Road, Pune -411041, Maharashtra, India. Abstract The aim of present work was to develop an accurate, precise, reproducible and economical UV spectrophotometric method for estimation of Ezetimibe. This method was based on area under curve of UV spectrum between 232 to 242 nm and validated as per ICH guideline Q2 (R1). The method has followed linearity in the range of 2-12µg/ml. The value of correlation coefficient was 0.999. Values for the intra-day and inter-day precision were satisfactory which indicated that method is precise. Results of the recovery studies (99.98% to 100.29 %) showed accuracy of the method. LOD and LOQ were calculated as 0.480 µg/ml and 1.851 µg/ml, respectively. The developed method can be used for routine estimation of Ezetimibe in bulk and tablet dosage forms. INTRODUCTION Ezetimibe is lipid lowering agent and it acts by inhibiting intestinal absorption of cholesterol and phytosterols. It interferes with a specific CH transport protein NPC1C1 in the intestinal mucosa and reduces absorption of both dietary and biliary cholesterol[2]. Chemically it is [(3R,4S) -1- (4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)3hydroxypropyl]-4(4-hydroxyphenyl)-2-azetidinone] (Fig. 1)[1]. It is a white, crystalline powder and it is freely to very soluble in ethanol, methanol, acetone and practically insoluble in water[3]. Literature survey revealed that some HPLC methods have been reported for estimation of Ezetimibe as single drug or in combination. [4,5,6,10,11]. Ezetimibe was also estimated by TLC [10]. Only few paper have been available in the literature using spectrophotometry for analysis of Ezetimibe as single drug & combination[7,8,9]. These techniques were simultaneous equation method, derivative spectroscopy, but none of these methods have utilized integration technique so far. In this context UV spectrophotometry further explore by using area under curve method for estimation of ezetimibe in bulk and tablet dosage form. 1 Figure 1 - Chemical structure of Ezetimibe. MATERIALS AND METHODS Apparatus and Instrumentation Shimadzu UV spectrophotometer 1800 with matched quartz cells was used and UV Prob Software, was employed for this work. Electronic balance (Shimadzu, AX 200, Japan) was used for weighing purpose. An Ultrasonic Cleaning Bath sonicator (Biosystem) was used for sonication. Galsswares (Borosil) used for this study were calibrated . Materials Active pharmaceutical ingredient (API) of Ezetimibe was procured as a gift sample from Genpharma International Pvt. Ltd, (Pune, India).Commercially available tablets (Zetia containing 10 mg of Ezetimibe ) were obtained from local pharmacy. Methanol (as a solvent) used was of AR-grade and was purchased from Merck India Ltd., Mumbai. Method Development Preparation of standard solution For the prepartion standard stock solution of Ezetimibe, 10 mg of API was accurately weighed and transferred to 10 ml of volumetric flask. The drug was dissolved with sonication in methanol for 10 min and volume was made up to the mark by using methanol. The standard stock solution (1000 μg/ml) of API was further diluted with methanol to get the concentration of 10 μg/ml. Selection of wavelength range Baseline correction was done by using methanol as blank. The standard solution of 10μg/ml of Ezetimibe was scanned between 400 nm to 200 nm in UV spectrophotometer. Wavelength range was selected around wavelength maxima (232 nm). Working standards were prepared between 2-12 μg/ml. Various wavelength range were tried around wavelength maxima (232nm). On the basis of linear relationship between area and corresponding concentration final range between 222-242 nm was selected. 2 Area under curve (Area calculation) This method involves calculation of integrated value of absorbance with respect to wavelength in indicated range. The area bounded by the curve and horizontal axis was calculated by are calculation processing item. The baseline was represented by horizontal axis. Preparation of calibration curve Working solutions of the concentration of 2, 4, 6, 8, 10 and 12 μg/ml were prepared from standard stock solution by further dilution with methanol. These solutions were scanned between 400 to 200 nm and area under curve (AUC) was integrated in the range of 222-242 nm. The calibration curve was plotted between area under curve against concentration (Fig. 3). Assay of Tablet Formulation Twenty tablets were weighed individually and average weight of tablet was calculated. These tablets were crushed and powdered by using glass mortar. The powder of tablet equivalent to 10 mg of Ezetimibe was accurately weighed, and transferred to a 100 ml of volumetric flask and diluted up to mark with methanol. This solution was filtered with Whatmann filter paper No.41 and the first few ml of filtrate was discarded. Further dilution of standard stock solution was done with same solvent to obtained 10 µg/ml of solution and subjected for UV analysis. This procedure was repeated in triplicate (Table 1). Method validation[12] The objective of validation of an analytical procedure is to demonstrate whether the procedure is suitable for its intended purpose. The proposed method was validated for various parameters such as Linearity, Accuracy, Precision, Limit of detection (LOD) and Limit of Quantitation (LOQ) as per the ICH Q2 (R1) guidelines. Linearity and Range The linearity was determined by using working standard solutions of Ezetimibe between 2-12 μg/ml. Area under curve was calculated in wavelength range of 222-242 nm. Calibration curve of Area under curve vs. Concentration was plotted and simple linear regression was performed (Figure 3). Regression equation and correlation coefficient were obtained. The range of solution has been decided according to statistical parameters of generated equation. 3 Method Precision Repeatability The precision of the method was checked by repeatedly injecting (n = 6) standard solutions of Ezetimibe (10 μg/ml). In the range of 222-242 nm area under curve of each of these solutions was measure Percentage relative standard deviation (RSD) was calculated (Table 2). Intermediate Precision (Reproducibility) The intra-day precision of the proposed method was determined by analyzing the corresponding responses 3 times on the same day and inter-day precision of the proposed method was determined by analyzing response on 3 different days over a period of 1 week for 3 different concentrations of standard solutions of Ezetimibe (5, 10 and 15 μg/ml). The results were reported in terms of relative standard deviation (RSD). The results are tabulated in Table 3. Accuracy The accuracy for the analytical procedure was determined at 80 %, 100 % and 120 % levels of standard solution. Area under curve was measured in the range of 222-242 nm. Results of above procedure were expressed in terms of % recoveries. Three determinations at each level were performed and % RSD was calculated. The results were tabulated in Table 4. Limit of Detection (LOD) and Limit of Quantitation (LOQ) Six sets of known concentrations (2-12 μg/ml) were prepared. Calibration curves were plotted for each set. LOD and LOQ were calculated using the formulae . 𝑆𝐷 𝑆 𝑆𝐷 𝐿𝑂𝑄 = 10 𝑆 𝐿𝑂𝐷 = 3.3 Where, SD is standard deviation of y-intercept of the calibration curves, S is mean slope of six calibration curves Result and Discussion For the determination of Ezetimibe in tablet dosage form An attempt was made to develop a simple and specific AUC spectrophotometric method. The generated regression equation was 222 222 ∫242 𝐴𝑑𝜆 = 0.136𝐶 + 0.057 (R2=0.999), Where ∫242 𝐴𝑑𝜆 is area under curve between 222 to 242 nm, C is concentration and R is correlation coefficient. R2 value is 0.999 which indicate that developed method was linear. As % R.S.D values for intraday as well interday precision were satisfactory therefore proposed method was found to be precise. It can be said that this method was accurate as drug at each of the 80 %, 100 % and 120 % levels showed 4 good recoveries (99.98 % to 100.29 %). The LOD and LOQ value were found to be 0.480 μg/ml and 1.851 μg/ml, respectively which shows that method is sensitive. The result of the analysis of formulation by the developed method was consistent with the label claim, highly reproducible and reliable. The method can be used for the routine analysis of the Ezetimibe in tablet dosage form. The validation parameters are summarised in Table 5. Area under curve Figure 2: UV spectrum of Ezetimibe (10 μg/ml) in methanol 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 y = 0.1362x + 0.0579 R² = 0.999 0 5 10 15 Concentration Figure 3: Calibration Curve of Ezetimibe (2-12 μg/ml) 5 CONCLUSION From these result it can be concluded that the proposed method was accurate, precise and consistent for determination of Ezetimibe in tablet dosage form. Validation of this method was carried out as per the ICH guidelines. It can be concluded that this this method can be used for routine estimation of Ezetimibe in pharmaceutical dosage forms ACKNOWLEDGEMENT The authors are grateful to Genpharma international Pvt. Ltd (Pune India) for providing API of Ezetimibe as gift sample and Dr. K.N. Gujar, Principal, Sinhgad College of Pharmacy, Vadgaon (Bk.), Pune for providing necessary facilities for this project. REFERENCES. 1. Susan B, Martha W, Blumetti FR. The Merck Index, 12th edition USA: Merck & Co; 1983. 2.Tripathi K.D Essentials of medical pharmacology, 6th edition: jaypee brother medical publisher;(2008). Page no. 618 3. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf 4. Baokar SB, Erande RS. and Shaikh SG. Analytical method development and validation for estimationof ezetimibe from tablet dosage form by using RP-HPLC , International Journal of Research in Pharmaceutical and Biomedical Sciences , 2011;2:833. 5. Chetan MB, Jane J and Subrahmanyam EVS. HPLC and Spectrophotometric estimation of ezetimibe, International Journal of Research in Pharmaceutical and Biomedical Sciences 2011;2: 241-244 6.Ramakrishna K, Pani Kumar AD, Venkat RY, Sunitha G, Rebecca SD, Bhandhavi S. Development of validated RP-HPLC method for the estimation of Ezetimibe in bulk drug and formulations, Research Journal of Pharmaceutical, Biological and Chemical Sciences 2011;2:815-821 7.Jain N, Jain R, Swami H, Pandey S. Spectrophotometric Method For Simultaneous Estimation of Simvastatin and Ezetimibe In Bulk Drug and its combined dosage form , Internatinal journal of pharmacy and pharmaceutical sciences 2009;1:170-175. 8. Gajjar AK, Shah VD. Simultaneous UV Spectrophotometric estimation of Rosuvastatin and Ezetimibe in their combined dosage forms, International Journal of Pharmacy and Pharmaceutical Sciences2010;2:131-138. 6 9.Shravya A, Chandan RS, Gurupadyya BM, Sireesha M. Spectrophotometric determination of Ezetimibe using MBTH reagent in pharmaceutical dosage form, International journal of research in ayurveda and Pharmacy 2011;2:521- 525. 10. Moghazy SM, Mohamed Abd AM, Marwa FM, and Nadia FY. Development and validation of HPLC, TLC and derivative spectrophotometric methods for the analysis of ezetimibe in the presence of alkaline induced degradation products, Journal of the Chinese Chemical Society 2009;56:360-367 11.Rajamanickam V, Rajasekaran A, Stephen B, Rathinaraj and Anandarajagopal K . Development and Validation of Analytical Methods for Simultaneous Estimation of Atorvastatin Calcium and Ezetimibe in Combined Dosage Form, World Applied Sciences Journal, 2010 ;12: 1424-1429. 12. ICH Harmonized-Tripartite Guidelines. Validation of Analytical Procedure: Text and Methodology Q2 (R1), November, 2005. 7 Table 1. Assay of Tablet Dosage Form. %RSD* Sr No Sample solution conc. Amount Found 1 10 101.23 2 10 100.93 3 10 100.89 niss *n=3 , % RSD – Relative standard deviation. Mean Amount Found (%) 101.01 0.183 8 Table 2. Repeatability Results for Ezetimibe. Drug Concentration of drug (μg/ml) %RSD* Ezetimibe *n=6 10 0.721 9 Table 3. Precision Results for Ezetimibe Drug Concentration of drug (μg/ml) % RSD intraday % RSD Interday Ezetimibe 5 10 15 0.376 0.373 0.380 0.390 0.401 0.391 10 Table 4. Accuracy Results for Ezetimibe Accuracy level Amount added (μg/ml % Recovery I (80%) II (100%) III (120%) 8 10 12 99.98 100.20 100.29 Mean % Recovery % RSD 100.15 0.159 11 Table 5. Summary of Validation Parameters. Parameter λ max Linearity range Regression Equation(y=mx+c) Slope (m) ± SD* Intercept (c) ± SD* Correlation Coefficient (R2) Precision (% R.S.D) Repeatability Intraday Interday Accuracy (Mean % Recovery LOD LOQ Result 232 2-12µg/ml y=0.136x + 0.057 0.136 0.057 0.999 0.721 0.376 0.394 100.15% 0.480 μg/ml 1.851 μg/ml 12