SUPPLEMENTARY MATERIAL New oxygenated himachalenes in
advertisement

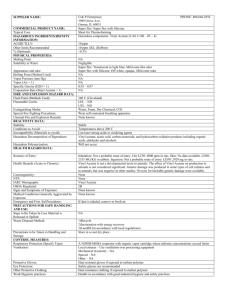
1 SUPPLEMENTARY MATERIAL New oxygenated himachalenes in male-specific odor of the Chinese windmill butterfly, Byasa alcinous alcinous Hisashi Ômuraa*, Taro Noguchia and Tatsuo Nehirab a Graduate School of Biosphere Science, Hiroshima University, Higashihiroshima 739-8528, Japan; bGraduate School of Integrated Arts and Sciences, Hiroshima University, Higashihiroshima 739-8521, Japan *Corresponding author. E-mail: homura@hiroshima-u.ac.jp Abstract Male adults of the Chinese windmill Byasa alcinous alcinous (Papilionidae) are well known to have a strong musk-like odor, in which two oxygenated himachalene compounds, together with six sesquiterpene hydrocarbons, were newly discovered. -Himachalen-4-yl acetate (1) was the predominant compound isolated from the solvent extract of the males. The structure of 1 was determined using MS and NMR, and its relative configuration was established as 1S*,4R*,6R* by NOE analysis with the help of quantum mechanical computation. Interestingly, the amount of 1 in males increased until 7 days after eclosion, suggesting that this compound is involved in sexual maturation for mating. Another new compound was identified as -himachalen-4-ol (2) by comparison with the retention time and mass spectrum of the hydrolysate of 1. Since males of other papilionid species have general volatiles omnipresent in plants and insects, the presence of species-specific volatiles in males is characteristic of B. alcinous alcinous. Keywords: Adult butterfly, Papilionidae, Byasa alcinous alcinous Male-specific volatiles, -Himachalene derivatives, Simulation-aided conformational analysis 2 Contents: Table S1. Chemical composition of sesquiterpene volatiles in the male extract of Byasa alcinous alcinous Table S2. Relevant distances between selected protons of -himachalen-4-yl acetate Table S3. Retention indices of sesquiterpene volatiles in the male extract of Byasa alcinous alcinous Figure S1. EI-Mass spectrum (70 eV) of -himachalen-4-yl acetate Figure S2. 1H NMR spectrum (400 MHz) of -himachalen-4-yl acetate Figure S3. 13C NMR spectrum (100 MHz) of -himachalen-4-yl acetate Figure S4. 1H–1H COSY spectrum (600 MHz) of -himachalen-4-yl acetate Figure S5. HMBC spectrum (600 MHz) of -himachalen-4-yl acetate Figure S6. 1H−1H COSY and the key HMBC correlations of -himachalen-4-yl acetate Figure S7. Selected NOE difference spectra (600 MHz) of -himachalen-4-yl acetate within the region between 2.0 and 6.0 ppm Figure S8. Selected NOE difference spectra (600 MHz) of -himachalen-4-yl acetate within the region between 0.9 and 5.5 ppm Figure S9. Experimental ECD spectrum of -himachalen-4-yl acetate ( in L mol-1 cm-1, c = 3.9 x 10-5 mol L-1) in acetonitrile Figure S10. Total ion chromatograms of the crude extract of male adult Byasa alcinous alcinous and the corresponding hydrolysate Figure S11. EI-Mass spectrum (70 eV) of -himachalen-4-ol Figure S12. 1H NMR spectrum (400 MHz) of -himachalen-4-ol Figure S13. 13C NMR spectrum (100 MHz) of -himachalen-4-ol Figure S14. EI-Mass spectra (70 eV) of -copaene, unknown C15H24, -himachalene, -himachalene, -himachalene, and -cadinene 3 Table S1. Chemical composition of sesquiterpene volatiles in the male extract of Byasa alcinous alcinous No.a Compound Male old Amount/individual (g, mean±SD) 3 -Copaene 3 day 0.28±0.16 4 unknown C15H24 3 day 0.37±0.23 5 -Himachalene 3 day 0.38±0.26 6 -Himachalene 3 day 0.41±0.32 7 -Himachalene 3 day 0.40±0.25 8 -Cadinene 3 day 0.46±0.30 2 -Himachalen-4-ol 3 day 0.90±0.83 1 -Himachalen-4-yl acetate 0 day 2.63±1.05 3 day 22.93±12.34 7 day 88.12±44.26 10 day 83.23±5.42 a The number of compound corresponds to the peak number in Figure 2. 4 Table S2. Relevant distances between selected protons of -himachalen-4-yl acetate All the stable conformations were obtained from DFT optimizations at B3LYP/6-31G(d) level under presence of chloroform with PCM method on Gaussian 09 after a standard conformational search with CONFLEX7/MMFF94S. The populations were estimated by the Boltzmann distribution at 298 K. Relative configuration (1S *,4R *,6R *) Conformer-ID c01 c02 c03 c04 (1S *,4S *,6R *) c01 c02 c03 (1S *,4S *,6S *) c01 c02 c03 c04 (1S *,4R *,6S *) c01 Experimental NOE Population (%) 12.7 10.3 59.0 18.1 21.3 10.0 68.7 24.7 14.7 27.5 33.1 100.0 H1 <-> H6 2.27 2.33 2.89 2.30 2.33 2.27 2.29 3.01 3.02 3.01 3.01 3.01 Observed Distance (angstroms) H4 <-> H6 H6 <-> H14 3.87 2.54 3.64 3.67 3.67 3.79 3.85 2.55 2.64 3.91 4.23 2.56 2.62 3.93 3.77 3.83 3.78 3.83 3.76 3.88 3.77 3.88 2.63 2.41 None Very weak H6 <-> H15 2.36 2.32 2.37 2.33 2.32 2.37 2.37 2.41 2.42 2.42 2.41 3.78 Observed 5 Table S3. Retention indices of sesquiterpene volatiles in the male extract of Byasa alcinous alcinous No.a Compound RIb measured HP-5MS DB-1 RI from litarature Authentic sample DB-1 1366 Reference Pala-Paul, J., Velasco-Negueruela, A., Perez-Alonso, M.J., Sanz, J. J . Chromatogr. A 2001, 923, 295-298. present in essential oil Ylang ylang oil (Cananga odorata ) Iranshahi, M., Amin, G., Sourmaghi, M.S., Shafiee, A., Hadjiakhoondi, A. Flavour Fragr . J . 2006, 21, 260-261. Pala-Paul, J., Perez-Alonso, M.J., Velasco-Negueruela, A., Vadare, J., Villa, A.M., Sanz, J., Brophy, J.J. J . Chromatogr . A 2005, 1074, 235-239. Pala-Paul, J., Brophy, J.J., Perez-Alonso, M.J., Usano, J., Soria, S.C. J . Chromatogr . A 2007, 1175, 289-293. Abella, L., Cortella, A.R., Velasco-Negueruela, A., Perez-Alonso, M.J. Pharm . Biol . 2000, 38, 3, 197-203. Atlas cedar oil (Cedrus atlantica ) Atlas cedar oil (Cedrus atlantica ) Atlas cedar oil (Cedrus atlantica ) Atlas cedar oil (Cedrus atlantica ) 3 -Copaene 1387 1366 4 unknown C15H24 1437 1409 5 -Himachalene 1472 1439 1438 6 -Himachalene 1499 1467 1461 7 -Himachalene 1522 1490 1495 8 -Cadinene 1524 1495 1501 2 -Himachalen-4-ol 1716 1662 1 -Himachalen-4-yl acetate 1830 1780 a The number of compoud corresponds to the peak number in Figure 2. b Retention index on a capillary column. c The components present in essential oils (Pranarôm International, Ghislenghien, Belgium) were used as authetic samples. c 6 Figure S1. EI-Mass spectrum (70 eV) of -himachalen-4-yl acetate [m/z (%)]: 262 (M+, 0.06), 220 (7), 202 (36), 187 (28), 173 (3), 159 (24), 146 (39), 145 (40), 133 (19), 132 (37), 131 (100), 119 (38), 117 (18), 115 (14), 105 (34), 93 (15), 91 (42), 79 (16), 77 (23), 60 (30), 55 (23), 53 (16), 45 (60), 43 (84), 41 (56). 7 (CH3CH2)2O ☓ (CH3CH2)2O ☓ CHCl3 ☓ Figure S2. 1H NMR spectrum (400 MHz) of -himachalen-4-yl acetate 8 CDCl3 Figure S3. 13C NMR spectrum (100 MHz) of -himachalen-4-yl acetate 9 Figure S4. 1H–1H COSY spectrum (600 MHz) of -himachalen-4-yl acetate 10 Figure S5. HMBC spectrum (600 MHz) of -himachalen-4-yl acetate 11 Figure S6. 1H−1H COSY and the key HMBC correlations of -himachalen-4-yl acetate 12 Figure S7. Selected NOE difference spectra (600 MHz) of -himachalen-4-yl acetate within the region between 2.0 and 6.0 ppm Trace 1: 1H-NMR spectrum (CDCl3) within the region between 2.0 and 6.0 ppm. Trace 2: NOE spectrum with saturation of the H-4 resonance (5.29 ppm). Trace 3: NOE spectrum with saturation of the H-6 resonance (2.53 ppm). Trace 4: NOE spectrum with saturation of the H-1 resonance (2.23 ppm). 13 Figure S8. Selected NOE difference spectra (600 MHz) of -himachalen-4-yl acetate within the region between 0.9 and 5.5 ppm Trace 1: 1H-NMR spectrum (CDCl3) within the region between 0.9 and 5.5 ppm. Trace 2: NOE spectrum with saturation of the H-4 resonance (5.29 ppm). Trace 3: NOE spectrum with saturation of the H-6 resonance (2.53 ppm). Trace 4: NOE spectrum with saturation of the H-1 resonance (2.23 ppm). 14 Figure S9. Experimental ECD spectrum of -himachalen-4-yl acetate ( in L mol-1 cm-1, c = 3.9 x 10-5 mol L-1) in acetonitrile The solution concentration was speculated from the UV absorption at 192 nm supposing the molar absorption coefficient 28,000 of a typical diene molecule. 15 Figure S10. Total ion chromatograms of the crude extract of male adult Byasa alcinous alcinous and the corresponding hydrolysate Peak 1: -himachalen-4-yl acetate, peak 2: -himachalen-4-ol. 16 Figure S11. EI-Mass spectrum (70 eV) of -himachalen-4-ol [m/z (%)]: 220 (M+, 2), 202 (34), 187 (31), 171 (10), 159 (31), 157 (20), 146 (40), 145 (54), 143 (16), 133 (21), 132 (47), 131 (100), 129 (21), 119 (46), 117 (24), 115 (24), 107 (16), 105 (51), 93 (21), 91 (50), 79 (18), 77 (27), 67 (14), 65 (17), 55 (26), 53 (19), 43 (20), 41 (61). 17 ☓ ☓ ☓ Figure S12. 1H NMR spectrum (400 MHz) of -himachalen-4-ol 18 ☓ Figure S13. 13C NMR spectrum (100 MHz) of -himachalen-4-ol ☓ 19 Figure S14. EI-Mass spectra (70 eV) of -copaene, unknown C15H24, -himachalene, -himachalene, -himachalene, and -cadinene The number of compound corresponds to the peak number in Figure 2.