Chapter 8 Formative 1. Which of the following is (are) true for
advertisement

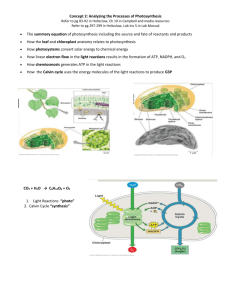
Chapter 8 Formative 1. Which of the following is (are) true for anabolic pathways? A) They do not depend on enzymes. B) They are highly regulated sequences of chemical reactions. C) They consume energy to build up polymers from monomers. D) They release energy as they degrade polymers to monomers. E) both B and C 2. Which of the following describe(s) some aspect of metabolism? A) synthesis of macromolecules B) breakdown of macromolecules C) control of enzyme activity D) A and B only E) A, B, and C 3. The mathematical expression for the change in free energy of a system is: G = H - T S. Which of the following is (are) incorrect? A) S is the change in entropy, a measure of randomness. B) H is the change in enthalpy, the energy available to do work. C) G is the change in free energy. D) T is the absolute temperature. E) both A and B 4. A chemical reaction that has a positive G is correctly described as A) endergonic. B) endothermic. C) enthalpic. D) spontaneous. E) exothermic. 5. Which of the following statements is (are) true about enzyme-catalyzed reactions? A) The reaction is faster than the same reaction in the absence of the enzyme. B) The free energy change of the reaction is the same as the reaction in the absence of the enzyme. C) The reaction always goes in the direction toward chemical equilibrium. D) A and B only E) A, B, and C 6. Which of the following statements regarding ATP is (are) correct? A) ATP serves as a main energy shuttle inside cells. B) ATP drives endergonic reactions in the cell by the enzymatic transfer of the phosphate group to specific reactants. C) The regeneration of ATP from ADP and phosphate is an endergonic reaction. D) A and B only E) A, B, and C 7. Which of the following statements is (are) true about enzyme-catalyzed reactions? A) B) C) D) E) The reaction is faster than the same reaction in the absence of the enzyme. The free energy change of the reaction is the same as the reaction in the absence of the enzyme. The reaction always goes in the direction toward chemical equilibrium. A and B only A, B, and C 8. According to the induced fit hypothesis of enzyme catalysis, which of the following is CORRECT? A) The binding of the substrate depends on the shape of the active site. B) Some enzymes change their structure when activators bind to the enzyme. C) A competitive inhibitor can outcompete the substrate for the active site. D) The binding of the substrate changes the shape of the enzyme's active site. E) The active site creates a microenvironment ideal for the reaction. The following questions are based on the reaction A + B 9. Which of the following terms best describes the reaction? A) endergonic B) exergonic C) anabolic D) allosteric E) nonspontaneous 10. Which of the following bests describes the reaction? A) negative G, spontaneous B) positive G, nonspontaneous C) positive G, exergonic D) negative G, endergonic E) G of zero, chemical equilibrium C + D shown in the figure below. Chapter 9 Formative 1. What is the reducing agent in the following reaction? Pyruvate + NADH + H+ a. b. c. d. e. Lactate + NAD+ oxygen NADH NAD+ lactate pyruvate 2. The immediate energy source that drives ATP synthesis by ATP synthase during oxidative phosphorylation is a. the oxidation of glucose and other organic compounds. b. the flow of electrons down the electron transport chain. c. the affinity of oxygen for electrons. d. the H+ concentration gradient across the inner mitochondrial membrane. e. the transfer of phosphate to ADP. 3. Which metabolic pathway is common to both fermentation and cellular respiration? a. the citric acid cycle b. the electron transport chain c. glycolysis d. synthesis of acetyl CoA from pyruvate e. reduction of pyruvate to lactate 4. In mitochondria, exergonic redox reactions a. are the source of energy driving prokaryotic ATP synthesis. b. are directly coupled to substrate-level phosphorylation. c. provide the energy to establish the proton gradient. d. reduce carbon atoms to carbon dioxide. e. are coupled via phosphorylated intermediates to endergonic processes. 5. The final electron acceptor of the electron transport chain that functions in oxidative phosphorylation is a. oxygen. b. water. c. NAD+. d. pyruvate. e. ADP. 6. When electrons flow along the electron transport chains of mitochondria, which of the following changes occurs? a. The pH of the matrix increases. b. ATP synthase pumps protons by active transport. c. The electrons gain free energy. d. The cytochromes phosphorylate ADP to form ATP. e. NAD+ is oxidized. 7. In the presence of a metabolic poison that specifically and completely inhibits all function of mitochondrial ATP synthase, which of the following would you expect? a. a decrease in the pH difference across the inner mitochondrial membrane b. an increase in the pH difference across the inner mitochondrial membrane c. increased synthesis of ATP d. increased oxygen consumption e. an accumulation of NAD+ 8. Cells do not catabolize carbon dioxide because a. its double bonds are too stable to be broken. b. CO2 has fewer bonding electrons than other organic compounds. c. CO2 is already completely reduced. d. CO2 is already completely oxidized. e. the molecule has too few atoms. 9. Which of the following is a true distinction between fermentation and cellular respiration? a. Only respiration oxidizes glucose. b. NADH is oxidized by the electron transport chain in respiration only. c. Fermentation, but not respiration, is an example of a catabolic pathway. d. Substrate-level phosphorylation is unique to fermentation. e. NAD+ functions as an oxidizing agent only in respiration. 10. Most CO2 from catabolism is released during a. glycolysis. b. the citric acid cycle. c. lactate fermentation. d. electron transport. e. oxidative phosphorylation. Chapter 10 Formative 1. The light reactions of photosynthesis supply the Calvin cycle with A) light energy. B) CO2 and ATP. C) H2O and NADPH. D) ATP and NADPH. E) sugar and O2. 2. Which of the following sequences correctly represents the flow of electrons during photosynthesis? A) NADPH O2 CO2 B) H2O NADPH Calvin cycle C) NADPH chlorophyll Calvin cycle D) H2O photosystem I photosystem II E) NADPH electron transport chain O2 3. Which of the following conclusions does not follow from studying the absorption spectrum for chlorophyll a and the action spectrum for photosynthesis? A) Not all wavelengths are equally effective for photosynthesis. B) There must be accessory pigments that broaden the spectrum of light that contributes to photosynthesis. C) The red and blue areas of the spectrum are most effective in driving photosynthesis. D) Chlorophyll owes its color to the absorption of green light. E) Chlorophyll a has two absorption peaks. 4. Cooperation of the two photosystems is required for A) ATP synthesis. B) reduction of NADP+. C) cyclic photophosphorylation. D) oxidation of the reaction center of photosystem I. E) generation of a proton-motive force. 5. In mechanism, photophosphorylation is most similar to A) substrate-level phosphorylation in glycolysis. B) oxidative phosphorylation in cellular respiration. C) the Calvin cycle. D) carbon fixation. E) reduction of NADP+. 6. In what respect are the photosynthetic adaptations of C4 plants and CAM plants similar? A) In both cases, only photosystem I is used. B) Both types of plants make sugar without the Calvin cycle. C) In both cases, an enzyme other than rubisco carries out the first step in carbon fixation. D) Both types of plants make most of their sugar in the dark. E) Neither C4 plants nor CAM plants have thylakoids. 7. Which of the following processes is most directly driven by light energy? A) creation of a pH gradient by pumping protons across the thylakoid membrane B) carbon fixation in the stroma C) reduction of NADP+ molecules D) E) removal of electrons from chlorophyll molecules ATP synthesis 8. Which of the following statements is a correct distinction between cyclic and noncyclic electron flow? A) Only noncyclic electron flow produces ATP. B) In addition to ATP, cyclic electron flow also produces O2 and NADPH. C) Only cyclic electron flow utilizes light at 700 nm. D) Chemiosmosis is unique to noncyclic electron flow. E) Only cyclic electron flow can operate in the absence of photosystem II. 9. Which of the following statements is a correct distinction between autotrophs and heterotrophs? A) Only heterotrophs require chemical compounds from the environment. B) Cellular respiration is unique to heterotrophs. C) Only heterotrophs have mitochondria. D) Autotrophs, but not heterotrophs, can nourish themselves beginning with CO2 and other nutrients that are inorganic. E) Only heterotrophs require oxygen. 10. Which of the following does not occur during the Calvin cycle? A) carbon fixation B) oxidation of NADPH C) release of oxygen D) regeneration of the CO2 acceptor E) consumption of ATP