H. Foronda, Phase Purity and Site Selection with La
advertisement

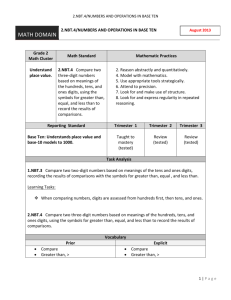
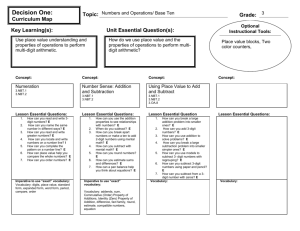
H. Foronda, Phase Purity and Site Selection with La Doping in NBT Phase Purity and Site Selection with Lanthanum Doping in Sodium Bismuth Titanate (Na0.5Bi0.5TiO3)-Piezoelectric Ceramic Humberto Foronda, Elena Aksel, and Jacob L. Jones Department of Materials Science and Engineering, University of Florida, Gainesville, FL Abstract Sodium bismuth titanate (NBT) is a lead-free piezoelectric material currently under study as a potential replacement to the commercially viable lead zirconate titanate (PZT). In order to improve the properties of the material, doping, or the small addition of ions to the material, can be used. The focus of this work is to examine the changes in the density and X-ray diffraction patterns of NBT with the addition of various lanthanum concentrations. Lanthanum was added in 0.5, 1.5, and 5 mol% to NBT using two different doping schemes. In one scheme, lanthanum was added to replace bismuth. In the other scheme, lanthanum was added to replace sodium. The addition of lanthanum with a reduction in sodium led a significant decrease in density, while density remained constant with the reduction in bismuth. Also, X-ray diffraction showed that with a doping level of 5%, an extra phase is forming using both doping schemes. These findings indicate that processing La-doped NBT may be more stable with a reduction in bismuth. piezoelectric ceramic is lead zirconate titanate (PZT). 1, 2 Its high piezoelectric properties and well established means of processing are reasons why it is the most commercially viable piezoelectric ceramic. 1, 2, 3 However, lead (Pb), which is harmful to the human body and the environment, is released into the atmosphere during processing of PZT. 4 Sodium bismuth titanate (NBT) is one of the potential replacements for PZT and was initially developed in the 1960s. 5, 6 NBT is a lead (Pb) free ceramic produced by the following reaction: Introduction Piezoelectricity is the ability of a material to electrically polarize in response to a mechanical stress and vice versa, mechanically strain in response to an applied electric field. 1 It is a rapidly developing topic in the field of materials science and engineering as piezoelectrics have many applications in industry. They are used in mobile phones, sonar, ultrasound, in fuel injectors of diesel engines. The most common commercially produced University of Florida | Journal of Undergraduate Research | Volume 11, Issue 2 | Spring 2010 1 H. Foronda, Phase Purity and Site Selection with La Doping in NBT where x is the percentage of added lanthanum (0.5%, 1.5%, or 5%). In Scheme A, bismuth is removed stoichiometrically in order to produce a vacancy which lanthanum could occupy. On the contrary, Scheme B is designed with the intention of placing lanthanum on the sodium site of the unit cell. The scheme is: ¼Bi2O3 + ¼Na2CO3+ TiO2→ Na0.5Bi0.5TiO3 + ¼CO2 (1) NBT has a perovskite structure7, as shown in Figure 1 below. Sodium and bismuth define the corners of the NBT unit cell, oxygen is located on each of faces and titanium is located in the center of the unit cell. Doping, which is the addition of a small amount of an element to the perovskite structure, is often used to alter the structure and properties of the material.8 This process has been studied to a great extent in PZT8-12; however, little work has been published on lead-free materials. Dopants, such as lanthanum and iron can be used to alter the structure and properties of NBT, for example its color, amount of oxygen vacancies, and its density.8 The purpose of this research is to better understand the structure of sodium bismuth titanate and the changes that take place with lanthanum doping. ¼Bi2O3 + ¼(1-3x) Na2CO3 +TiO2 + 0.5xLa2O3 → Na0.5(1-3x)Bi0.5TiO3 + ¼CO2 (3) In this case sodium is removed stoichiometrically in order to produce a vacancy for lanthanum to occupy. The first step in processing begins with mixing stoichiometric amounts of powders (according to schemes A and B above). The powders used to process NBT are sodium carbonate (99.5% purity, Alfa Aesar), bismuth oxide (99.975% purity, Alfa Aesar), and titanium dioxide (99.85% purity, Alfa Aesar). The dopant lanthanum oxide (99.99% purity, Alfa Aesar) was also added according to the two reaction schemes. The powders are then suspended in ethanol to form a slurry and ball milled with yttria stabilized zirconia for approximately twenty four hours. The ball milled slurry is dried and calcined at 800ºC for two hours using heating and cooling rates of 4 and 5°C/min, respectively, to form La doped Na0.5Bi0.5TiO3. Afterwards, an organic polyvinyl alcohol binder is added to the powders to aid with pressing and the mixture is ground to pass through a 200 micron sieve. It is then packed in a 10 mm diameter pressing die and pressed using a uniaxial press for one minute at 300 MPa. The green pellets are then sintered in a furnace at 1100°C for up to one hour, using heating and cooling rates of 4 and 5°C/min, respectively, to reduce porosity and consolidate the powders into a solid body. Density of the sintered ceramics was measured using the Archimedes method. The weight of the pellets in air and submerged in water was recorded and used to calculate the density with the following equation: Figure 1- Pseudo-cubic representation of the NBT Unit Cell Experimental Lanthanum doped NBT was processed using two different reaction schemes, each with the intention of placing lanthanum on a specified site within the NBT unit cell. The first reaction (Scheme A) was designed for lanthanum to sit on the bismuth site of the unit cell. The reaction scheme used is: ¼(1-x) Bi2O3 + ¼Na2CO3 + TiO2+ 0.5xLa2O3 → Na0.5Bi0.5(1-x)LaxTiO3 + ¼CO2 (2) University of Florida | Journal of Undergraduate Research | Volume 11, Issue 2 | Spring 2010 2 H. Foronda, Phase Purity and Site Selection with La Doping in NBT Density Wair Denisty fluid Wair W fluid (4) Results and Discussion The average density measurements for the various pellets are shown in table 1. It is clear from the table that as the percentage of lanthanum increases in Scheme A, the densities remain unchanged. The average densities in this case are 5.90, 5.91, and 5.74 g/cm3 and they are all within 5% of each other. However, in Scheme B, the density decreases as the percentage of lanthanum added increases. With 0.5% lanthanum, the average density of a sintered pellet is 5.92 g/cm3. When 5% lanthanum is added, the average density drops to 4.9 g/cm3. The theoretical density of undoped NBT is 5.99 g/cm3.8 To calculate the theoretical density of samples produced using the various doping schemes, the mass of the unit cell was calculated assuming that all of the added lanthanum went to the intended site (i.e., sodium or bismuth) and that the volume of the unit cell is constant. The percent of theoretical density is found from the ratio of the measured density to the calculated theoretical density. The density for Scheme A remains at approximately 98% of the theoretical density while in Scheme B it drops from 98.2% to 79.4% with increased lanthanum content. Where Wair is weight of the pellet in air, W fluid is the weight of the pellet in fluid, and Denisty fluid is the density of the fluid. Finally, an Inel CPS120 X-ray diffractometer (shown below in Figure 2) was used to measure X-ray diffraction patterns of the processed materials. Six minute diffraction patterns of each prepared pellet were measured to examine its phase purity. Figure 2 - Inel CPS120 X-ray diffractometer Table 1- Density Measurements of doped NBT ceramics Lanthanum (%) Site Density (g/cm3) Percent of Theoretical Density (%) 0 - 5.84 97.5 0.5 Bi 5.90 98.3 1.5 Bi 5.91 98.8 5 Bi 5.74 97.1 0.5 Na 5.92 98.2 1.5 Na 5.64 93.1 5 Na 4.91 79.4 University of Florida | Journal of Undergraduate Research | Volume 11, Issue 2 | Spring 2010 3 H. Foronda, Phase Purity and Site Selection with La Doping in NBT Figures 3 and 4 show the measured XRD patterns. Figure 3 corresponds to Scheme A, where lanthanum is intended to replace bismuth while Figure 4 corresponds to Scheme B, where lanthanum is intended to replace sodium. The samples produced with 0.5% lanthanum in both schemes do not show the presence of any extra phases, as the peaks in the pattern correspond to the undoped NBT perovskite structure. As more of the dopant is added to the samples, extra peaks begin to appear which indicate undesired secondary phases. This is clear when 5% dopant is used as there are several extra peaks in both figures. The extra peaks are marked with red arrows in both figures 3 and 4 for the 5% samples. The phase purity of the samples decreases with an increase in the percentage of lanthanum. Figure 3-Diffraction patterns of NBT-La produced according to scheme A, with La on Bi site Figure 4 – Diffraction patterns of NBT-La produced according to scheme B, with La on Na site University of Florida | Journal of Undergraduate Research | Volume 11, Issue 2 | Spring 2010 4 H. Foronda, Phase Purity and Site Selection with La Doping in NBT 4. E. Cross, “Materials Science: Lead-free at Last,” Nature, 432, 24-25 (2004). Conclusion In this work, lanthanum doped sodium bismuth titanate was successfully produced using solid state processing. Two different doping schemes were used (Schemes A and B) for the addition of lanthanum. In Scheme A the addition of La is compensated for with an equal reduction in Bi while in Scheme B it is compensated for with a reduction in Na. Density measurements of the prepared ceramics show that using Scheme A the density remains relatively unchanged while in Scheme B it decreases greatly with an increase in La addition. This behavior indicated that the La dopant may be more stable on the Bi site. Also, XRD measurements of the processed ceramics show only NBT perovskite peaks at low concentrations of La in both schemes. However, as the amount of La added increased to 5%, extra phases appeared in both doping schemes. This indicates that adding 5% La to NBT using either doping scheme does not form a stable perovskite. 5. M. D. Maeder, D. Damjanovic, and N. Setter, “Lead Free Piezoelectric Materials,” J. Electroceram., 13, 385-392 (2004). 6. G. A. Smolensky, V. A. Isupov, A. I. Agranovskaya, and N.N. Krainik, “New Ferroelectrics of Complex Composition,” Soviet Physics-Solid State, 2, 2651-2654 (1961). 7. G. O. Jones, P. A. Thomas, “Investigation of the structure and phase transitions in the novel A-site substituted distorted perovskite compound Na0.5Bi0.5TiO3,” Acta Crystallographica Section B: Structural Science, 58, 168-178 (2002). 8. D. Viehland, “Effect of Uniaxial Stress Upon the Electromechanical Properties of Various Piezoelectric Ceramics and Single Crystals,” Journal of the American Ceramic Society, 89, 775-785 (2006). 9. Kulcsar, F. “Electromechanical properties of lead titanate zirconate ceramics modified with certain three- or five-valent additions,” J. Am. Ceram. Soc., 42, 343-349 (1959). Acknowledgements The authors gratefully acknowledge support for this work from the University Scholars Program at the University of Florida, the Research Experience in Materials (REM) program in the Department of Materials Science at the University of Florida, and the National Science Foundation award DMR0746902. 10. Berlincourt, D. Piezoelectric ceramic compositional development. J. Acoust. Soc. Am., 91, 3034-3040 (1992). 11. Zhang, X.L.; Chen, X.; Cross, L.E.; Schulze, W.A. “Dielectric and piezoelectric properties of modified lead titanate zirconate ceramics from 4.2 to 300K,” J. of Mat. Sci., 18, 968-972 (1983). References 1. B. Jaffe, W. R. Cook, and H. Jaffe, Piezoelectric Ceramics, Academic, London, 1971. 12. Berlincourt, D. “Piezoelectric ceramics: Characteristics and applications,” J. Acoust. Soc. Am, 70, 1586-1595 (1981). 2. N. Setter ed., Piezoelectric Materials in Devices. Lausanne: EPFL, 2002. 3. G. H. Haertling, “Ferroelectric Ceramics: History and Technology,” J. Am. Ceram. Soc., 82, 797-818 (1999). University of Florida | Journal of Undergraduate Research | Volume 11, Issue 2 | Spring 2010 5