Catalytic Converter Fact Sheet
advertisement

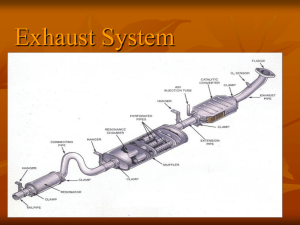
Catalytic Converter Fact Sheet Background Information: First made in 1975 by John J. Mooney and Dr. Carl D. Keith. It was dedicated to stop air pollution A device used to reduce the toxicity of emissions from an internal combustion engine Works by using a specific catalyst to inspire a chemical reaction Convert toxic by-products from engines into less toxic substances In order to control emissions, amount of fuel used is carefully controlled Air-to-fuel ratio close to the stoichiometric point Ideal ratio of air to fuel. At this ratio, all of the fuel will be burned using all the oxygen in the air. For gasoline, ratio = 14.7 : 1. Meaning each mole of gasoline, 14.7 moles of air will be combusted Not constant, can easily be changed Preheating can increase efficiency Catalytic Converters in Diesel engines aren’t as efficient. Diesel engines are cooler run cooler engines. Even though it take away the toxins, but a major producer of CO2 Scientists are trying to find a way to conquer both problems without any compromises How it Works: The main emissions of a car are: N2(g), CO2(g) and H2O(g) Not considered to be health hazards, but CO2 contributes to global warming Since combustions are never perfect, smaller amounts of more harmful toxins are released. The catalytic converter is made to reduce them CO: poisonous gas that is colourless and odourless Hydrocarbons: smog, evaporated unburned fuel, harmful NO & NO2: contributor to smog and acid rain 2 different kinds of catalysts are needed. Reduction catalyst and oxidation catalyst Consist of ceramic structure coated with a metal catalyst. Usually platinum, rhodium and/or palladium. Gold may also be used. Cheaper than those materials and reduce 40% more toxins Expose maximum surface area of catalyst to the exhaust stream while minimizing catalyst required Modern cars have three-way catalytic converters. 3 regulated commissions. First stage, reduction catalyst. Uses Pt & Rb to reduce NO & NO2 NO N2 + O2 2NO2 N2 + 2O2 When NO or NO2 contacts the catalyst, catalyst rips N atoms out of molecule, freeing the O2. The N then bond with other N to for N2 Second stage, oxidation catalyst. Uses Pt & Pd to reduce hydrocarbons and CO 2CO + O2 2CO2 CxH2x+2 + [(3x+1)/2]O2 → xCO2 + (x+1)H2O Reduces unburned hydrocarbons and CO by burning them over a catalyst Third stage, control system. Monitors the exhaust stream, control fuel injection system. A sensor will tell the engine computer how much oxygen is in the exhaust. Oxygen can be increased or decreased by adjusting the air-to-fuel ratio. It makes sure that the engine is running close to stoichiometric point, and it also make sure there are sufficient oxygen to combust the hydrocarbon and CO Bibliography Who Invented the Catalytic Converter? | eHow.com. (n.d.). eHow | How To Do Just About Everything! | How To Videos & Articles. Retrieved November 29, 2010, from http://www.ehow.com/facts_5901250_invented-catalytic-converter_.html Nice, K. (n.d.). HowStuffWorks "Car Engine Emissions". Howstuffworks "Auto ". Retrieved November 29, 2010, from http://auto.howstuffworks.com/catalytic-converter1.htm Catalytic converter - Wikipedia, the free encyclopedia. (n.d.). Wikipedia, the free encyclopedia. Retrieved November 30, 2010, from http://en.wikipedia.org/wiki/Catalytic_converter Anglin, D. L. (2010). Catalytic Converter. Grolier Multimedia Encyclopedia. Retrieved November 30, 2010, from Grolier Online http://gme.grolier.com/article?assetid=0054700-0