Anemias Complete List
advertisement
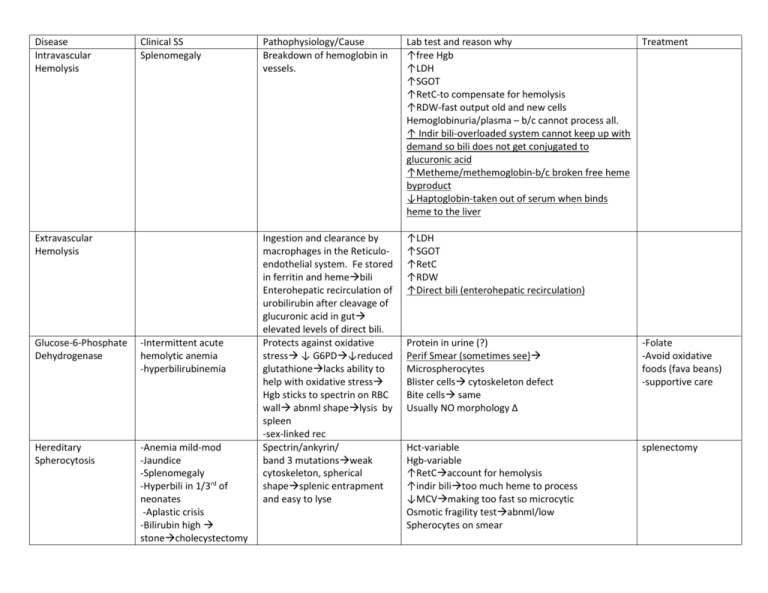
Disease Intravascular Hemolysis Clinical SS Splenomegaly Extravascular Hemolysis Glucose-6-Phosphate Dehydrogenase -Intermittent acute hemolytic anemia -hyperbilirubinemia Hereditary Spherocytosis -Anemia mild-mod -Jaundice -Splenomegaly -Hyperbili in 1/3rd of neonates -Aplastic crisis -Bilirubin high stonecholecystectomy Pathophysiology/Cause Breakdown of hemoglobin in vessels. Lab test and reason why ↑free Hgb ↑LDH ↑SGOT ↑RetC-to compensate for hemolysis ↑RDW-fast output old and new cells Hemoglobinuria/plasma – b/c cannot process all. ↑ Indir bili-overloaded system cannot keep up with demand so bili does not get conjugated to glucuronic acid ↑Metheme/methemoglobin-b/c broken free heme byproduct ↓Haptoglobin-taken out of serum when binds heme to the liver Ingestion and clearance by macrophages in the Reticuloendothelial system. Fe stored in ferritin and hemebili Enterohepatic recirculation of urobilirubin after cleavage of glucuronic acid in gut elevated levels of direct bili. Protects against oxidative stress ↓ G6PD↓reduced glutathionelacks ability to help with oxidative stress Hgb sticks to spectrin on RBC wall abnml shapelysis by spleen -sex-linked rec Spectrin/ankyrin/ band 3 mutationsweak cytoskeleton, spherical shapesplenic entrapment and easy to lyse ↑LDH ↑SGOT ↑RetC ↑RDW ↑Direct bili (enterohepatic recirculation) Treatment Protein in urine (?) Perif Smear (sometimes see) Microspherocytes Blister cells cytoskeleton defect Bite cells same Usually NO morphology Δ -Folate -Avoid oxidative foods (fava beans) -supportive care Hct-variable Hgb-variable ↑RetCaccount for hemolysis ↑indir bilitoo much heme to process ↓MCVmaking too fast so microcytic Osmotic fragility testabnml/low Spherocytes on smear splenectomy PK-Pyruvate Kinase Deficiency Autoimmune Hemolytic Disorders COLD/INTRAvascular Variable chronic anemia Extravascular hemolysis Splenomegaly Gallstones Aplastic crisis Dark Urine Acute/Chronic anemia Pallor/Jaundice Splenectomy s.times Problempyruvate problem ↓ATP production ↑2,3-BPG and mis-shapen RBCssplenic destruction IgG and IgMactivates C5-9 complementRBC damage by complement ↑RetCaccount for hemolysis NO morphology Δ SAME FOR EXTRA ↓ -Folate -Spenectomy-s.times -transfusions in severe cases ↑RetC ↑Bili Hemoglobinuria Spherocytes Bite cells Direct Coombs (test for IgG, C3D, C4D-bound to RBC) + Complement (C3D, C4D) Indirect Coombs (test for IgG and Complement-free in plasma) + complement Autoimmune Hemolytic Disorders WARM/EXTRAvasc. Dark Urine Acute/Chronic anemia Pallor/Jaundice Splenectomy s.times IgG only Opsonizing Macrophages and spleen affected so that the lysis is in the spleen and outside the blood system ↑RetC ↑Bili Hemoglobinuria Spherocytes Bite cells Direct Coombs (test for IgG, C3D, C4D-bound to RBC) + IgG (sometimes weak complement) Indirect Coombs (test for IgG and Complement-free in plasma) + IgG Iron Deficiency Fatigue Pallor Pain with exercise SOB Tachycardia Muscle problems Neurological problems Dietary Absorption problems Inflammation/Infection Cytokine production Microcytic and Hypochromic ↓Hgb-no iron to bind heme ↓Hct-less and smaller RBC’s bc no iron ↓ferritin-iron stores used up ↓RetC-because no iron to make RBC’s ↓serum iron ↑TIBC-bc capacity is increased due to no binding ↑RDW Perif Smear Spherocytes, fragmented cells, target cells Oral iron salts (150200 mg tid) IV/IM iron. 1st see serum Fe respond RetC nmlizes, Hgb nml after 7- 10 days. Continue treatment until ferritin stores returned and MCV and RDW are nml (which will take 100 days or so until abnml RBC’s have been removed) Methemoglobinemia -Cyanosis without accompanying ↓ in arterial PP -Brown-blood due to Fe+3 without change when exposed to O2 -In severe casesbrady, respire depression, convulsions, acidosis. Acquired -free radicals (NO, H2O2) -Drugs (benzocaine) Hereditary -homozygous def of cytochrome-b-5-reductase (prevents reduction of Fe+3Fe+2) -Hemoglobin M (mutation of chains that inhibits reduction of Fe+3) Anemia of Chronic Disease/Inflammatio n -Variable anemia -Underlying inflammatory disease -Renal insufficiency -Thyroid disorder -Adrenal insufficiency -fever -arthritis -swelling (infections) Inability to use iron stores and decrease EPO production result from inflammatory cytokines: -TNF/IL-1↓iron mobilization/ EPO production -INFγ/βinhibits erythropoisis -Hepcidin (induced by cytokines) ↑ Fe storage, ↓duod abs, block Fe release from macrophages Lead Based Anemia -Personality changes -irritability -weakness -wt loss -abdominal pain and vomiting -Pb inhibits synthesis of protoporphyrin ring↓heme Sideroblastic Anemia Variable anemia Impaired production of Acquired Remove drug or chemical causing disease Hereditary Methylene Blue -Hgb 8-12 -Usually Normocytic/ normochromic microcytic /mild hypochroma ↓RetC –reticulocytopenia b/c cannot get iron since sequestered due to presence of hepcidin. ↑Ferritin-due to increased/sequestration of iron(DIFF from Iron defic anemia) ↓TIBC (DIFF from IDA) ↓Serum Iron (like IDA) ↓Transferrin saturation (as with IDA) ↓EPO for degree of anemia (espec with renal disease. But usually not seen until <40% renal function) ↓MCV = Microcytic/hypochromic because Pb is preventing production of porphyrin ring ↓RetC-because no heme to make iron so cannot make RBC’s ↑Lead levels Basophilic stippling-aggregates of RNA(also seen with macrocytic anemia of B12 and Folate) -Treat underlying condition to ↓cytokines and inflammation -Give iron -Give EPO (especially with renal disease) -Hormone replacement of endocrine disease ↓MCV = B6-s.times works 1. REMOVE source of lead 2. Chelation protoporphyrin ring or incorporation of Fe into heme accumulation of iron in mitochondria Ring sideroblasts Required for synthesis of methionine from homocysteineprecursors for DNA synthesiseffect erythropoisis in BM. So essentially it is a disease of ↓DNA synthesis Causes -Vegan Diet-rare cause -IF deficient = Pernicious Anemia (mal-abs of B12) -autoimmune destruction of parietal cells that secrete IF -Loss of ilial receptors-surgery, IBD TcII deficiency Microcytic/hypochromic Pappenheimer bodies (ppt iron in mitochondriaring around nucleus) ↑MVC = Macrocytic/Normochromic (because increase in size as waiting for DNA to be made-arrest in S phase ↓RBC ↓RetC-because no B12 to make cells Hypersegmented neutrophils-classical sign of B12 or folate deficiency. ↑RDWanisocytosis -Nuclear-Cytoplasmic Asynchrony M:E ratioE predominance ↑Homocysteine levels ↑methylmalonic acid ↑ to nml serum folate* ↓B-12 levels (s.times predictive) Shillings Test: If abnml then add IF and see if corrects. If normal then not B-12 abs problem Transfusions Tx of other underlying cause (Cu deficiency, EtOHism, drugs) B-12 injections Oral replacement ↑dosesabs via mass action Watch for other immune diseases -Rapid response to treatment and slowly resolving CNS indicates accurate dx. B-12 Deficiency Slow (months) onset CNS changes-cognitive dysfunction and emotional changes Numbness, tingling, loss of fine sensations Proprioception Ataxia CNS changes may be permanent. Folate Deficiency Quick Onset (weeks) Required for synthesis of methionine from homocysteineprecursors for DNA synthesiseffect erythropoisis in BM. So essentially it is a disease of ↓DNA synthesis Causes Insufficient dietary intake*1⁰ Mal-absparasitic infection (disrupts enterohepatic recirc) EtOH consumption excess ↑MVC = Macrocytic/Normochromic (because ↓RBC ↓RetC-because no B12 to make cells Hypersegmented neutrophils ↑RDWanisocytosis -Nuclear-Cytoplasmic Asynchrony M:E ratioE predominance ↑Homocysteine levels ↓serum folate ↓methylmalonic acid Folate 1mg/d PO/IV/IM Rapid recovery of CBC. Sickle Cell Anemia Chronic Hemolytic Anemia Sickling cells are actually “sticky” which leads to vessel ↑RetC-to compensate for hemolysis (except during aplastic crisis) 1. BM transplant w/ HLA matched sibling Aplastic crisis - ↓RetC Pain crisis – Acute chest pain, multi-organ failure, priapism, bone infaction. damage, endothelial remodeling and vessel narrowing. This microvessel damage affects all organs (lungs, retina, kidneys, spleen, CNS) Hb H (Barts) Mod-severe anemia Infant Barts bodies = γ-4 H-bodies in adult Hb EE Mild Anemia Mild splenomegaly Extramedullary Hematopoiesisfrontal bossing, hair on ends, enlarged mandible etc Severe anemia Extramedullary Hematopoiesis 2/3rds have abnml endocrine problems (hypothyroid, gonad problems etc) --/-α limited binding to gamma chain during in-utero development and then βagglutination during life. Hb E is an unstable globin chain ↓amount for heme production Cooley’s β-thal Major 2 severely abnml β-globin chains ↑ Levels of Hb F (10-90%) ↑WBC/platelets due to BM response ↑RDW-increase production and constantly changing shape of the sickled cell ↓MCV – Microcytosis (Sβ⁰/Sβ+) Heme C crystals = red rods in RBC’s (SC disease) ↑Hb Fetal (2-30%) ↑LDH, AST (released from lysed RBC) ↑indirect bilirubin-due to hemolysis ↑Total bilirubin Perif Smear Schistocytes Polychromasia-blue colored cells Anisocytosis (RDW effect) Howell Jolly bodies-occur in pt w/out functional spleen due to injury/occlusion from sickled cellssplenic sequestration. ↓MCV – bc low α chains and agglutination of βchains whichBarts ↓MCHC-because low levels of heme since less produced ↓MCV = Microcytic ↓MCHC-because low levels of heme Target cells = Mexican hat = Excess of RBC plasma membrane due to ↓in heme content to RBC itself (RBC is not filled up so takes on this shape) ↓MCV = Microcytic ↓MCHC-because low levels of heme since less produced Target cells = Mexican hat = Excess of RBC plasma membrane is 90% 2. Hydroxyurea ↑Hb F 3. Transfusions – decrease pain crises extend life. Transfusions s.times No transfusions ALWAYS transfusions Always chelation tx Hydroxyurea=↑HbF (via increasing gamma chains that can bind with the excess α-chains.







