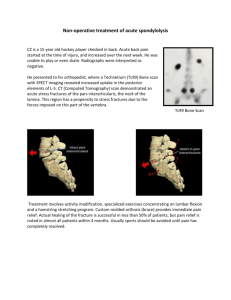
Precautions
advertisement

eMedicine Specialties > Nephrology > Glomerular Diseases Glomerulonephritis, Poststreptococcal Duvuru Geetha, MD, MRCP, Assistant Professor of Medicine, Department of Renal Medicine, Bayview Medical Center, Johns Hopkins University Updated: Sep 22, 2009 Introduction Background Acute glomerulonephritis is characterized by the sudden appearance of hematuria, proteinuria and red blood cell casts in the urine, edema, and hypertension with or without oliguria. It can follow streptococcal infections. This illness was first recognized as a complication of the convalescence period of scarlet fever in the 18th century. A link between hemolytic streptococci and acute glomerulonephritis was recognized in the 20th century. A diagram of a nephron is shown in the image below. Diagram of a nephron. Although the incidence of poststreptococcal glomerulonephritis has declined in the United States, it continues to have high incidence in other parts of the world, especially in areas with tropical climates where skin infections are common.[1,2 ] Pathophysiology Poststreptococcal glomerulonephritis follows infection with only certain strains of streptococci, designated as nephritogenic. The offending organisms are virtually always group A streptococci. Acute poststreptococcal glomerulonephritis (APSGN) follows pyodermatitis with streptococci M types 47, 49, 55, 2, 60, and 57 and throat infection with streptococci M types 1, 2, 4, 3, 25, 49, and 12. Although many morphologic, clinical, and serologic features suggest that APSGN is an immune complex disorder, the precise nature of the antigen-antibody interaction is undefined. APSGN is believed to be an immune-mediated disease, in which an immune complex containing a streptococcal antigen is deposited in the affected glomeruli. The size of glomerular basement membrane (GBM) pores and the molecular size of the streptococcus-Ig complex are also important determinants. The molecular size of the streptococcus-Ig complex is about 15 nm (10 nm for streptococcus group A and 5 nm for immunoglobulin). The GBM pore sizes in children and adults are 2-3 nm and 4-4.5 nm, respectively. Therefore, the immune complex molecule can be more easily rodded into the glomerulus in children than in adults and, thus, may explain the increased frequency of APSGN in children compared to that in adults. The 2 antigens isolated from nephritogenic streptococci are under investigation in APSGN. These include the cationic cysteine protease streptococcal pyrogenic exotoxin B and nephritisassociated streptococcal plasmin receptor, which is a plasmin-binding protein with glyceraldehyde phosphate dehydrogenase (also known as presorbing antigen or PA-Ag).[3 ]These fractions have an affinity for glomeruli and have been shown to induce specific, long-lasting antibody responses in biopsy specimens from patients with APSGN. The relevance of exotoxin B and glyceraldehyde phosphate dehydrogenase was evaluated in the same renal biopsy and serum samples of patients with well-defined APSGN. Glomerular deposits of and antibody response to exotoxin B were more consistently present in APSGN than were deposits of and antibody response to glyceraldehyde phosphate dehydrogenase. Antibodies to exotoxin B and PA-Ag are elevated in the majority of patients with APSGN. Intravenous injections of PA-Ag produce acute glomerulonephritis in animals. Antibodies to PAAg are found in 30 of 31 patients with APSGN but are low or absent in those with uncomplicated streptococcal infection or in patients with rheumatic fever. PA-Ag is also known to activate the alternate pathway of the complement cascade, which happens to be preferentially activated in persons with APSGN. The observation that some patients may only have C3 deposition may relate to this mechanism. In addition to streptococcal antigens, rheumatoid factor, cryoglobulins, and antineutrophil cytoplasmic serum antibodies are present in some of these patients. The pathogenic significance of this autoimmune response is not defined. There are also host susceptibility factors. In one study, HLA-DRB1*03011 was reported to be found at a significantly higher frequency in 32 unrelated patients with APSGN as compared to 380 healthy individuals.[4 ] Frequency United States The incidence of clinically detectable glomerulonephritis during an epidemic is up to 10% of children with pharyngitis and 25% of children with impetigo. One study reported a change in the epidemiology of APSGN and found that pharyngitis has replaced impetigo as the predominant cause of APSGN.[5 ] International APSGN can occur sporadically or epidemically. Incidence seems to be decreasing in the United States and Europe, but sporadic cases of the disease continue to be reported from all over the world. The prevalence of nephritis varies considerably among persons with sporadic infections with nephritogenic streptococci. The reason for this variability is not known. Epidemic poststreptococcal glomerulonephritis occurs mainly in developing countries in areas such as Africa, the West Indies, and the Middle East. Reasons for this changing epidemiology relate to the nutritional status of the community, the more liberal use of antibiotic prophylaxis, and possibly, the change in the nephritogenic potential of streptococci. Among epidemic infections with nephritogenic streptococci, the apparent clinical attack rate is 10-12%.[1,2 ] Mortality/Morbidity Early death is extremely rare in children (<1%) but is significantly more common in adults (25%). This is secondary to congestive heart failure and azotemia. Congestive heart failure is more common in adults (43%) than in children (<5%). Nephrotic-range proteinuria is more common in adults (20%) than in children (4-10%). Approximately 83% of adults have azotemia, compared to 25-40% of children. Race No racial predilection is recognized. Sex Clinical cases of APSGN are twice as common in males than in females. If subclinical disease is considered, both sexes are affected equally. The familial incidence rate is nearly 40%, but no genetic marker has been identified. Age This condition typically affects children aged 2-12 years. A large series reported that 5% are younger than 2 years and 10% are older than 40 years. Clinical History A history suggestive of preceding streptococcal infection may include a preceding infective episode such as pharyngitis, tonsillitis, or pyoderma. This is the sine qua non for the diagnosis of APSGN. Latent period o A latent period always occurs between the streptococcal infection and the onset of signs and symptoms of acute glomerulonephritis. o In general, the latent period is 1-2 weeks after a throat infection and 3-6 weeks after a skin infection. o The onset of signs and symptoms at the same time as pharyngitis (also called synpharyngitic nephritis) is more likely to be immunoglobulin A (IgA) nephropathy rather than APSGN. Dark urine (brown-, tea-, or cola-colored) o This is often the first clinical symptom. o Dark urine is caused by hemolysis of red blood cells that have penetrated the glomerular basement membrane and have passed into the tubular system. Periorbital edema o The onset of puffiness of the face or eyelids is sudden. It is usually prominent upon awakening and, if the patient is active, tends to subside at the end of the day. o In some cases, generalized edema and other features of circulatory congestion, such as dyspnea, may be present. o Edema is a result of a defect in renal excretion of salt and water. o The severity of edema is often disproportionate to the degree of renal impairment. Nonspecific symptoms o These can include general malaise, weakness, and anorexia and are present in 50% of patients. o Approximately 15% of patients complain of nausea and vomiting. Physical Acute nephritic syndrome o o Edema o Acute nephritic syndrome presenting as edema, hematuria, and hypertension with or without oliguria is the most frequent presentation of APSGN. Approximately 95% of clinical cases have at least 2 manifestations, and 40% have the full-blown acute nephritic syndrome. Edema is present in 80-90% of cases, and it is the presenting complaint in 60% of cases. o Compromised intraglomerular blood flow due to glomerular hypercellularity results in progressive encroachment on the cross-sectional area of the glomerular capillaries. o This leads to reduced blood flow that manifests as low fractional excretion of sodium and concentrated urine. This salt and water retention leads to edema. Hypertension o Hypertension occurs in 60-80% of cases and is more common among elderly individuals. o In 50% of cases, the hypertension can be severe; however, more often it is transient, with normalization of blood pressure upon restoration of the glomerular filtration rate, loss of edema, and normalization of plasma volume. o If hypertension persists, it is more indicative of the progression to a more chronic stage or that the disease is not poststreptococcal glomerulonephritis. o Hypertension is thought to be the result of excessive salt and water retention. o Despite excessive sodium retention, the plasma levels of atrial natriuretic peptide are increased. In this condition, this suggests that the kidneys are unresponsive to atrial natriuretic peptide. o Plasma renin activity is usually low, and studies by Parra et al have shown that an inhibition of angiotensin-converting enzyme could be an effective short-term treatment for this low-renin hypertension.[6 ] o Hypertensive encephalopathy occurs in no more than 5-10% of patients. Usually, clinical improvement occurs without any neurological sequelae. Oliguria o This is present in 10-50% of cases, and, in 15%, urine output is less than 200 mL. o Oliguria is indicative of the severe crescentic form of the disease. o It is often transient, with diuresis occurring within 1-2 weeks. Hematuria o This is present universally. o In 30% of cases, gross hematuria is present. Left ventricular dysfunction o Left ventricular dysfunction with or without hypertension or pericardial effusion may be present during the acute congestive and convalescent phases. o In rare cases, persons with APSGN can show signs of pulmonary hemorrhage. o In a study of 28 pediatric patients with APSGN, Taskesen et al found that on clinic admission, the plasma levels of NT-proBNP (an N-terminal peptide left over when the prohormone for brain natriuretic peptide [proBNP] is cleaved to produce active BNP) were higher in these patients than in the 26 healthy children making up the control group.[7 ]Moreover, the NT-proBNP levels were significantly higher in 6 patients with APSGN who were found to have left ventricular dysfunction than they were in the patients with APSGN in whom no ventricular dysfunction was diagnosed. The authors suggested that in some patients with APSGN, determination of NT-proBNP levels may prove helpful in the assessment of left ventricular volume overload and cardiac function. Causes Poststreptococcal glomerulonephritis follows infection with only certain strains of streptococci designated as nephritogenic. The offending organisms are virtually always group A streptococci. APSGN follows pyodermatitis with streptococci M types 47, 49, 55, 2, 60, and 57 and throat infection with streptococci M types 1, 2, 4, 3, 25, 49, and 12. Differential Diagnoses Antiglomerular Basement Membrane Disease Cryoglobulinemia Glomerulonephritis, Membranoproliferative Glomerulonephritis, Nonstreptococcal Associated With Infection Nephritis, Lupus Other Problems to Be Considered Bacterial endocarditis Essential cryoglobulinemia IgA nephropathy Microscopic polyarteritis Shunt nephritis Visceral abscess Workup Laboratory Studies Evidence of preceding streptococcal infection o Antibody titers to extracellular products of streptococci are positive in more than 95% of patients with pharyngitis and 80% of patients with skin infections. o The antistreptolysin (ASO), antinicotinamide adenine dinucleotidase (anti-NAD), antihyaluronidase (AHase), and anti–DNAse B are commonly positive after pharyngitis, and anti–DNAse B and AHase titers are more often positive following skin infections. o ASO titers are frequently used to document streptococcal infection, but a more sensitive test is the streptozyme test, which tests antibodies to ASO, anti–DNAse B, AHase, and anti-NAD. o Studies suggest that the relatively unavailable antizymogen titer test is superior to both anti–DNAse B and ASO titers. o Antizymogen titers that are 2 dilutions higher than the mean in healthy controls are reported to have a sensitivity of 88% and a specificity of 85% in the diagnosis of streptococcal infection in patients with glomerulonephritis. o High antibody titers to glyceraldehyde phosphate dehydrogenase are also found in persons with acute poststreptococcal glomerulonephritis (APSGN). o In general, the antibody titers are elevated at 1 week, peak at 1 month, and fall toward preinfection levels after several months. Elevated BUN and creatinine values o This reflects the decrease in the glomerular filtration rate that occurs in the acute phase. o The elevations are usually transient. o Their failure to normalize within several weeks or months indicates that the patient may not have a true APSGN and suggests seeking an alternative diagnosis. o Patients who have the crescentic form of glomerulonephritis have rapid deterioration and, often, incomplete recovery of renal function. Serologic findings o Low serum complement levels indicative of an antigen-antibody interaction are a universal finding in the acute phase of APSGN. o Most patients have marked depression of serum hemolytic component CH50 and serum concentrations of C3. o The activation of the alternative pathway of the complement system is thought to be responsible for the hypocomplementemia. o In some patients, the levels of C2 and C4 may also be decreased, but to a lesser extent, suggesting that both classic and alternate pathways of the complement system are activated. o In most uncomplicated cases, the complement levels return to normal in 6-8 weeks. Prolonged hypocomplementemia suggests an alternative diagnosis. o Occasionally, low complement levels persist for 3 months. o The level of reduction of serum complement levels does not have any prognostic significance. o Circulating immune complexes and cryoglobulins are found in 60% of cases, and rheumatoid factor is found in 43% of cases. Urinalysis o Results are always abnormal. o Hematuria and proteinuria are present in all cases. o Urine sediment has red blood cells, red blood cell casts, white blood cells, granular casts, and, rarely, white blood cell casts. o Dysmorphic red blood cells indicative of glomerular hematuria can usually be detected by performing phase-contrast microscopy. o Red blood cell casts are best detected in first, early-morning urine specimens examined by the physician immediately after the patient voids. o Hematuria usually resolves within 3-6 months but may persist as long as 18 months. o o o o o Microscopic hematuria may be present in patients in whom the disease has otherwise clinically resolved. Proteinuria may be mild or so severe that it causes nephrotic syndrome. Approximately 5-10% of patients with APSGN have nephrotic-range proteinuria. Proteinuria usually disappears in 6 months. A mild increase in urinary protein excretion is present in 15% at 3 years and 2% at 10 years. Patients with nephrotic-range proteinuria in the acute phase or persistent heavy proteinuria have a worse prognosis. This is often associated with an evolution to a garlandlike pattern of immune deposits as the disease progresses. Imaging Studies Chest radiographs may show findings of congestive heart failure. Renal ultrasound images usually reveal normal-sized kidneys bilaterally. Procedures APSGN is often a clinical diagnosis and requires the detection of glomerulonephritis and evidence of preceding streptococcal infection. Atypical features in the early phase that suggest the need for renal biopsy include the following: o Absence of the latent period between streptococcal infection and acute glomerulonephritis o Anuria o Rapidly deteriorating renal function o Normal serum complement levels o No rise in antistreptococcal antibodies o Extrarenal manifestations of systemic disease o No improvement or continued decrease in the glomerular filtration rate at 2 weeks o Persistence of hypertension beyond 2 weeks Atypical features in the recovery phase that mandate a renal biopsy include the following: o Failure of glomerular filtration rate to normalize by 4 weeks o Persistent hypocomplementemia beyond 6 weeks o Persistent microscopic hematuria beyond 18 months o Persistent proteinuria beyond 6 months Histologic Findings Pathologic findings of changes in gross appearance o The kidneys are symmetrically enlarged to approximately 25-50% of normal. o They are pale in appearance, and the cut surfaces bulge because of interstitial edema. o The glomeruli may stand out as reddish or gray translucent dots. o The cut surfaces may have tiny red speckles caused by red blood cells in the lumen of the Bowman space and tubules. Light microscopy o The most striking finding is hypercellularity of the glomeruli. All glomeruli are affected (diffuse) and usually to an approximately equal degree. The glomerular tufts are larger than normal, and the cells are more numerous. o The cell types typically present include endothelial and mesangial cells and migrant inflammatory cells, which include polymorphonuclear leukocytes and monocytes. o Polymorphonuclear leukocytes are present in large numbers, hence the term exudative glomerulonephritis. o Necrosis in the glomerular tuft is not typically found. o The individual lobules are wider than usual and may have a clubbed appearance. o Generally, the glomerular capillary walls are not thick. o In some patients, crescent formation may be found, but usually, only a small percentage of glomeruli are affected by crescents. o The tubules are normal in the majority of cases. o When proteinuria is present, hyaline droplets (protein reabsorption droplets) may be present in the proximal convoluted tubules. o In patients with severe exudative glomerulonephritis, polymorphonuclear leukocytes may be present in the lumen. o The degree of interstitial involvement is variable. The interstitial areas show edema and infiltration with polymorphonuclear leukocytes and mononuclear cells. The arteries and arterioles are normal. Immunofluorescence o In biopsy samples taken in the first 2-3 weeks of illness, deposits of immunoglobulin G and C3 in a diffuse granular pattern are present along the glomerular capillary wall and mesangium. o Immunoglobulin M may be present in small amounts. Significant amounts of IgA suggest an alternative diagnosis. o Sorger et al have described 3 different patterns of immunofluorescence called the garland pattern, the starry sky pattern, and the mesangial pattern.[8 ] o The starry sky pattern is an irregular, finely granular pattern with small deposits often situated on the glomerular basement membrane overlying the mesangium. This pattern is often seen in the early phase of the disease. o The starry sky pattern may turn into the mesangial pattern, which is characterized by granular deposition of C3 with or without immunoglobulin G. It seems to be most closely related to a resolving pattern. o In approximately 25% of patients, the deposits are large and densely packed and aggregate into a ropelike or garlandlike pattern. These correspond to the humps on the subepithelial side of the glomerular capillary wall seen with electron microscopy. These types of deposits may persist for months and may be associated with the persistence of proteinuria and the development of glomerulosclerosis. Electron microscopy o Many of the ultrastructural changes confirm the findings from light microscopy evaluations. o The number of endothelial, mesangial, and infiltrating inflammatory cells is increased. o o The glomerular basement membrane is usually normal in thickness and contour, although occasionally patchy thickening may be noted. The most consistent and classic diagnostic finding is the presence of glomerular subepithelial electron-dense immune-type deposits, often referred to as humps. The deposits are discrete and are commonly found on the part of the glomerular basement membrane overlying the mesangium. Treatment Medical Care Symptomatic therapy is recommended for patients with acute poststreptococcal glomerulonephritis (APSGN), and it should be based on the clinical severity of the illness. The major goal is to control edema and blood pressure. During the acute phase of the disease, restrict salt and water. o If significant edema or hypertension develops, administer diuretics. o Loop diuretics increase urinary output and consequently improve cardiovascular congestion and hypertension. For hypertension not controlled by diuretics, usually calcium channel blockers or angiotensin-converting enzyme inhibitors are useful. For malignant hypertension, intravenous nitroprusside or other parenteral agents are used. Indications for dialysis include life-threatening hyperkalemia and clinical manifestations of uremia. Restricting physical activity is appropriate in the first few days of the illness but is unnecessary once the patient feels well. Steroids, immunosuppressive agents, and plasmapheresis are not generally indicated. A renal biopsy is indicated for patients with rapidly progressive renal failure. If the biopsy findings show evidence of crescentic glomerulonephritis with more than 30% of the glomeruli involved, a short course of intravenous pulse steroid therapy is recommended (500 mg to 1 g/1.73 m2 of methylprednisone qd for 3-5 d). However, no controlled clinical trials have evaluated such therapy. Long-term treatment with steroids or immunosuppressives is not recommended. Specific therapy for streptococcal infection is an important part of the therapeutic regimen. o Treat patients, family members, and any close personal contacts who are infected. o Throat cultures should be performed on all these individuals. Treat with oral penicillin G (250 mg qid for 7-10 d) or with erythromycin (250 mg qid for 7-10 d) for patients allergic to penicillin. o This helps prevent nephritis in carriers and helps prevent the spread of nephritogenic strains to others. Patients with skin infections must practice good personal hygiene. This is essential. During epidemics, recommend that high-risk individuals, including close contacts and family members, receive empirical prophylactic treatment. Surgical Care Surgical care is not indicated. Consultations Nutritionist or dietitian Nephrologist Diet Low-salt diet - Two grams of sodium per day Fluid restriction - One liter per day Activity Restricting physical activity is appropriate in the first few days of the illness but is not necessary once the patient feels well. Medication Therapy for patients with APSGN is symptomatic in nature and depends on the clinical severity of the illness. The major aims are to control the edema and blood pressure. During the acute phase of the disease, salt and water should be restricted. If significant edema or hypertension develops, diuretics should be administered. Loop diuretics increase urinary output and consequently improve cardiovascular congestion and hypertension. For hypertension not controlled by diuretics, calcium channel blockers or angiotensin-converting enzyme inhibitors are generally useful. For malignant hypertension, intravenous nitroprusside or other parenteral agents are used. The indications for dialysis include life-threatening hyperkalemia and clinical manifestations of uremia. Steroids, immunosuppressive agents, and plasmapheresis are not generally indicated. In patients with rapidly progressive renal failure, a renal biopsy is indicated. If the biopsy findings show evidence of crescentic glomerulonephritis with more than 30% of the glomeruli involved, a short course of intravenous pulse steroid therapy is recommended (500 mg to 1 g/1.73 m2 of methylprednisone qd for 3-5 d). However, no controlled clinical trials have evaluated such therapy. Long-term treatment with steroids or immunosuppressives is not recommended. Diuretics Used to control edema and circulatory congestion. Furosemide (Lasix) Increases excretion of water by interfering with chloride-binding cotransport system, which, in turn, inhibits sodium and chloride reabsorption in ascending loop of Henle and distal renal tubule. Dose must be individualized to patient. Depending on response, administer at increments of 20-40 mg, no sooner than 6-8 h after the previous dose, until desired diuresis occurs. When treating infants, titrate with increments of 1 mg/kg/dose until a satisfactory effect is achieved. Dosing Adult 20-40 mg PO/IV q6-8h initial; maximum dose can be titrated carefully to 600 mg/d Pediatric 1-2 mg/kg PO/IV; doses > 6 mg/kg not recommended Interactions Metformin decreases concentrations; interferes with hypoglycemic effect of antidiabetic agents and antagonizes muscle-relaxing effect of tubocurarine; auditory toxicity appears to be increased with coadministration of aminoglycosides; hearing loss of varying degrees may occur; anticoagulant activity of warfarin may be enhanced when taken concurrently; increased plasma lithium levels and toxicity are possible when taken concurrently Contraindications Documented hypersensitivity; hepatic coma, anuria, and state of severe electrolyte depletion Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Perform frequent serum electrolyte, carbon dioxide, glucose, creatinine, uric acid, calcium, and BUN determinations during first few months of therapy and periodically thereafter Calcium channel blockers In specialized conducting and automatic cells in the heart, calcium is involved in the generation of the action potential. Calcium channel blockers inhibit movement of calcium ions across the cell membrane, depressing both impulse formation (automaticity) and conduction velocity. Amlodipine (Norvasc) Relaxes coronary smooth muscle and produces coronary vasodilation, which, in turn, improves myocardial oxygen delivery. Benefits nonpregnant patients with systolic dysfunction, hypertension, or arrhythmias. Can be used during pregnancy if clinically indicated. Dosing Adult 5-20 mg PO qd Pediatric Not established Interactions Fentanyl may increase hypotensive effects; may increase cyclosporin levels; H2 blockers (cimetidine) may increase toxicity Contraindications Documented hypersensitivity Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Adjust dose in renal/hepatic impairment; may cause lower extremity edema; allergic hepatitis has occurred but is rare Angiotensin-converting enzyme inhibitors Decrease aldosterone secretion. Captopril (Capoten) Prevents conversion of angiotensin I to angiotensin II, a potent vasoconstrictor, resulting in lower aldosterone secretion. Dosing Adult 25 mg PO bid/tid; not to exceed 150 mg tid Pediatric Not established; use only if other measures to control blood pressure have not been effective Interactions NSAIDs may reduce hypotensive effects; may increase digoxin, lithium, and allopurinol levels; rifampin decreases levels; probenecid may increase levels; hypotensive effects may be enhanced when given concurrently with diuretics Contraindications Documented hypersensitivity; renal impairment Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus Precautions Pregnancy category D in second and third trimesters; caution in renal impairment, valvular stenosis, or severe congestive heart failure Enalapril (Vasotec) Competitive inhibitor of angiotensin-converting enzyme. Reduces angiotensin II levels, decreasing aldosterone secretion. Clinical response usually observed within 15 min of administration. Dosing Adult 10-40 mg PO qd in 1-2 divided doses Hypertensive emergencies: 1.25 mg IV q6h; can increase to 5 mg q6h Pediatric Not established Interactions NSAIDs may reduce hypotensive effects; may increase digoxin, lithium, and allopurinol levels; rifampin decreases levels; probenecid may increase levels; hypotensive effects may be enhanced when given concurrently with diuretics Contraindications Documented hypersensitivity Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus Precautions Pregnancy category D in second and third trimesters; caution in renal impairment, valvular stenosis, or severe congestive heart failure Vasodilators Used to treat hypertensive emergencies. Vasodilators reduce SVR, which, in turn, may allow forward flow, improving cardiac output. Nitroprusside (Nitropress) Produces vasodilation and increases inotropic activity of heart. At higher dosages, may exacerbate myocardial ischemia by increasing heart rate. Should not be used to treat compensatory hypertension (arteriovenous shunt or coarctation of aorta). Dosing Adult 0.25-10 mcg/kg/min IV infusion Pediatric 1-8 µmol/kg/min IV Interactions Effects are additive when administered with other hypotensive agents Contraindications Documented hypersensitivity; subaortic stenosis, idiopathic hypertrophic and atrial fibrillation or flutter Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Caution in increased intracranial pressure, hepatic failure, severe renal impairment, and hypothyroidism; in renal or hepatic insufficiency, levels may increase and can cause cyanide toxicity; sodium nitroprusside has the ability to lower blood pressure and thus should be used only in patients with mean arterial pressures >70 mm Hg Hydralazine (Apresoline) Decreases systemic resistance through direct vasodilation of arterioles. Dosing Adult 10-20 mg IV q4-6h Pediatric 0.1-0.2 mg/kg IV q4-6h Interactions MAOIs and beta-blockers may increase toxicity; pharmacologic effects may be decreased by indomethacin Contraindications Documented hypersensitivity; mitral valve rheumatic heart disease Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Has been implicated in myocardial infarction; caution in possible coronary artery disease; caution in slow acetylators for fear of causing drug-induced lupus Follow-up Further Inpatient Care In the acute phase, admit for observation and treatment of hypertension and congestive heart failure. Admit for monitoring and to initiate dialysis (when indicated) if renal function progressively worsens. Further Outpatient Care Monitor blood pressure every month for 6 months and then every 6 months thereafter. Monitor BUN and serum creatinine levels every 3 months after the acute phase for 1 year and then yearly after that. Check serum complement levels at 6-8 weeks to make sure they have returned to normal. Check urine for hematuria and proteinuria every 3-6 months. Inpatient & Outpatient Medications Most patients do not require any medications after the acute phase. In the acute phase, diuretics may be needed to control edema and congestive heart failure. Antihypertensives may be needed in the chronic phase if the patient's blood pressure remains high. Transfer Transfer may be necessary if renal biopsy facilities are not available and the diagnosis is in doubt or if rapidly progressive renal failure develops. Transfer may be necessary if azotemia worsens and dialysis facilities are not available on site. Deterrence/Prevention The patient and any family member or close personal contact should have a throat culture. Treatment with penicillin G or erythromycin (if allergic to penicillin) helps prevent nephritis in carriers and helps prevent the spread of nephritogenic strains to others. Patients with skin infections must pay close attention to personal hygiene. Epidemics should prompt empirical prophylactic treatment for high-risk individuals (family and close personal contacts). Complications Complications in the acute phase include the following: o Congestive heart failure o Azotemia o Early death secondary to congestive heart failure and azotemia Complications in the chronic phase include the following: o Nephrotic-range proteinuria o Chronic renal insufficiency and end-stage renal disease Prognosis In children, the immediate prognosis is excellent.[1 ] In elderly patients who have congestive heart failure or azotemia in the early phase, early mortality rates can be as high as 25%. The long-term prognosis is debatable. o Fewer than 1% of children have elevated serum creatinine values after 10-15 years of follow-up. o Adults who develop massive proteinuria often have the garlandlike pattern of immune deposits. Their prognosis is worse; approximately 25% progress to chronic renal failure. Patient Education Patients with skin infections should know the importance of personal hygiene. In epidemics, all close personal contacts and family members should be told to seek medical attention for prophylactic treatment of streptococcal infections. For excellent patient education resources, visit eMedicine's Kidneys and Urinary System Center and Ear, Nose, and Throat Center. Also, see eMedicine's patient education articles Blood in the Urine and Strep Throat. Miscellaneous Medicolegal Pitfalls Failure to follow up on the natural course of the disease o Make sure serum complement levels have returned to normal at 6-8 weeks. o Make sure hematuria and proteinuria resolve or improve and do not get worse. Failure to make the correct diagnosis o A risk of missing alternative diagnoses in atypical cases exists if a renal biopsy is not performed. o See Procedures for details on indications for renal biopsy. Special Concerns Patients with subclinical nephritis outnumber those with overt nephritis by a ratio ranging from 4-10:1. Careful evaluation of abnormal urinalysis results may help reveal a diagnosis of poststreptococcal glomerulonephritis. eMedicine Specialties > Pediatrics: General Medicine > Nephrology Acute Poststreptococcal Glomerulonephritis Rajendra Bhimma, MB, ChB, MD, DCH (SA), FCP (Paeds)(SA), MMed (Natal), Associate Professor of Pediatrics, Principal Specialist, Department of Pediatrics and Child Health, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, South Africa Updated: Jan 7, 2010 Introduction Background Acute glomerulonephritis (AGN) is a disease characterized by the sudden appearance of edema, hematuria, proteinuria, and hypertension. It is a representative disease of acute nephritic syndrome in which inflammation of the glomerulus is manifested by proliferation of cellular elements secondary to an immunological mechanism. Acute poststreptococcal glomerulonephritis (APSGN) results from infection by nephritogenic streptococci. The latter are defined as organisms that are cultured from patients who develop acute poststreptococcal glomerulonephritis. The concept of nephritogenic streptococci was initially advance by Seegal and Earl, who noted that rheumatic fever and acute poststreptococcal glomerulonephritis (both nonsuppurative complications of streptococcal infections) did not simultaneously occur in the same patient and differ in geographical location. Acute poststreptococcal glomerulonephritis occurs predominantly in males and often completely heals, whereas patients with rheumatic fever often experience relapsing attacks. Acute poststreptococcal glomerulonephritis is now known to follow infection by group A beta hemolytic streptococci. The M and T proteins in the bacterial wall have been used for characterizing streptococci; M types 1, 2, 4, 12, 18, 25, 49, 55, 57, and 60 may have nephritogenic potential. These may cause skin or throat infections, but specific M types, such as 49, 55, 57, and 60, are most commonly associated with skin infections. In addition, nontypeable group A streptococci are frequently isolated from the skin or throat of patients with glomerulonephritis, representing presumably unclassified nephritogenic strains. The overall risk of developing acute poststreptococcal glomerulonephritis after infection by these nephritogenic strains is about 15%. The risk of nephritis may also be related to the M type and the site of infection. The risk of developing nephritis infection by M type 49 is 5% if it is present in the throat. This risk increases to 25% if infection by the same organism in the skin is present. Pathophysiology Most forms of acute poststreptococcal glomerulonephritis are mediated by an immunologic process. Both cellular and humoral immunity is important in the pathogenesis of acute poststreptococcal glomerulonephritis. Humoral immunity in acute poststreptococcal glomerulonephritis is presumed to be mediated by the in situ formation of nephritogenic streptococcal antigen-antibody complexes and circulating immune complexes . The most widely proposed mechanism for the development of acute poststreptococcal glomerulonephritis is that nephritogenic streptococci produce proteins with unique antigenic determinants. These antigenic determinants have a particular affinity for sites within the normal glomerulus. Following release into the circulation, these antigens bind to these sites within the glomerulus. Once bound to the glomerulus, they activate complement directly by interaction with properdin. Glomerular-bound streptococcal antibodies also serve as fixed antigens and bind to circulating antistreptococcal antibodies forming immune complexes. Complement fixation via the classical pathway leads to generation of additional inflammatory mediators and recruitment of inflammatory cells. The 2 major nephritogenic antigens that have been identified are zymogen, a precursor of exotoxin B called SPEB (also described as nephritis strain-associated protein [NSAP]), and nephritis plasmin binding protein (NAPlr). NSAP was detected in renal biopsies of patients with acute poststreptococcal glomerulonephritis but not in other forms of AGN or rheumatic fever . NSAP has antigenic, biochemical, and structural similarities to streptokinase from group C streptococcal organisms, binds to plasmin, and is a plasminogen activator. The molecule has been isolated and purified and has subunit of 46kDa. Yoshizawa et all isolated NAPlr and noted that this antigen was present in 100% of the early biopsies in glomeruli of patients with acute poststreptococcal glomerulonephritis.[1 ]The molecular weight of the purified antigen NAPlr is 43kD, and the internal amino acid sequence was found to be homologous to plasmin receptor (Plr) and glyceraldehyde-3phosphatedehydrogenase(GAPDH) of group A streptococcal strain 64/14. A mechanism for acute poststreptococcal glomerulonephritis that has been proposed is that soluble, released NAPlr binds to glomeruli and provide a mechanism to capture plasmin activated by streptokinase. The activated plasmin bound to NAPlr associates with the glomerular basement membrane and mesangium. Bound plasmin can cause tissue destruction by direct action on the glomerular basement membrane or by indirect activation of procollagenases and other matrix metalo-proteinases. NAPlr can also activate the alternate complement pathway, leading to accumulation of polymorphonuclear cells and macrophages and local inflammation. Also, the in situ–formed and circulating immune complexes can readily pass through the altered glomerular basement membrane and accumulate on the subepithelial space as humps. A mechanism for acute poststreptococcal glomerulonephritis proposed by Yoshizawa is shown in the image below. A schematic representation of the proposed mechanism for acute poststreptococcal glomerulonephritis (APSGN). C = Activated complement; Pl = Plasmin; NAPlr = Nephritis-associated plasmin receptor; SK = Streptokinase; CIC = Circulating immune complex. Other nonimmmune complex mediated mechanisms have been proposed for the development of acute poststreptococcal glomerulonephritis. Firstly, a role for delayed-type hypersensitivity has been implicated in the pathogenesis of this disease. Early in the course, resident endothelial and mesangial cells are predominantly proliferated, and this is accompanied by infiltration with polymorphonuclear leukocytes and monocytes. Macrophages are effector cells that cause resident cellular proliferation. The infiltration of macrophages in the glomeruli is mediated by complement-induced chemotaxis and, most likely, by an antigen-specific event related to delayed-type hypersensitivity mediated by helper/inducer T cells. Secondly, streptococcal M proteins and pyrogenic exotoxins can act as superantigens. These cause a marked expansion of T cells expressing specific T-cell receptor B-chain variable gene segments. Massive T-cell activation, with release of T-cell–derived lymphokines such as interleukin 1 and interleukin 6. Thirdly, autologous immunoglobulin G (IgG) in acute poststreptococcal glomerulonephritis becomes antigenic and elicits an anti-IgG rheumatoid factor response, leading to formation of cryoglobulins. Cryoglobulins, rheumatoid factors, and other autoimmune phenomena occur in acute poststreptococcal glomerulonephritis and are thought to play a role in the pathogenesis of the disease together with streptococcal superantigens. Although renal biopsies are now rarely indicated in children with typical poststreptococcal acute glomerulonephritis, they have contributed substantially to the understanding of this disease. Irrespective of the degree of severity of the initial inflammatory response, the histologic picture is consistent and specific. As observed by light microscopy, glomerular changes are generalized and diffuse. The glomerular tufts usually appear enlarged and swollen, and a moderate-tomarked increase in proliferation of mesangial and epithelial cells is present. Polymorphonuclear leukocytes are also often observed as part of the inflammatory process. In persons with the most severe disease, the glomeruli appear bloodless because of the associated edema of the capillary walls, which impedes glomerular perfusion. A direct correlation exists between the severity of the histologic process and the clinical manifestations of the disease during the acute phase and possibly the prognosis. Granular deposits of IgG and C3 are typically found when the specimen is studied by immunofluorescent microscopy; other immunoglobulins (Igs) and fibrinogen are often observed. Electron microscopy of renal tissue from patients with poststreptococcal acute glomerulonephritis usually reveals subepithelial electron-dense deposits (humps). In most patients with moderate-to-severe AGN, a measurable reduction in volume of glomerular filtrate (GF) is present, and the capacity to excrete salt and water is usually diminished, leading to expansion of the extracellular fluid (ECF) volume. The expanded ECF volume is responsible for edema and, in part, for hypertension, anemia, circulatory congestion, and encephalopathy. Frequency United States Over the last 2-3 decades, the incidence of acute poststreptococcal glomerulonephritis has declined in the United States as well as other countries, such as Japan, Central Europe, and Great Britain. However, in recent years, a slight increase in the incidence of the disease has been reported. The actual incidence of the disease is still unknown. The decline in the incidence of the disease may be due to the improvement in living conditions with less crowding. However, other factors, including decreased prevalence or infectivity of the nephritogenic streptococci, may also have contributed to the decline in incidence. International A decline in the incidence of acute poststreptococcal glomerulonephritis in developed and developing countries has been reported over the last 2-3 decades. As many as 50% of cases may be subclinical; thus, the true incidence of the disease is unknown. Nevertheless, acute poststreptococcal glomerulonephritis continues to have a wide distribution as indicated by reports of the disease from all over the world. Because a high percentage of persons affected with acute poststreptococcal glomerulonephritis have mild disease and are asymptomatic (estimates of the ratio of asymptomatic to symptomatic patients vary from 2:1-3:1), the actual incidence of the disease is not known. Less-crowded living conditions may have contributed to the apparent decline in the incidence of acute poststreptococcal glomerulonephritis over the past few decades; however, other factors, including decreased prevalence or infectivity of the nephritogenic streptococci, may also have contributed to the decline. The recently observed increase in incidence is more difficult to explain. Acute poststreptococcal glomerulonephritis usually occurs as sporadic cases, but epidemic outbreaks have taken place in communities with densely populated dwellings that have poor hygienic conditions with a high incidence of malnutrition, anemia, and intestinal parasites. In certain regions, epidemics may occur in cyclical outbreaks every 5-7 years for unknown reasons. A strong seasonal variation is also noted; sporadic acute poststreptococcal glomerulonephritis following upper respiratory infection, pharyngitis, and tonsillitis is more common in winter and spring in temperate areas, whereas skin infections are commonly found to precede acute poststreptococcal glomerulonephritis in the more tropical and subtropical areas, with a peak incidence during summer and autumn. The disease is more frequent in children aged of 2-12 years. However, in most large series, 510% of patients are older than 40 years, and 5% are younger than 2 years. Although a male dominance is noted in symptomatic cases, when subclinical and clinical disease is taken into account, the rates are the same in males and females. Mortality/Morbidity The ultimate prognosis in persons with acute glomerulonephritis depends largely on the severity of the initial insult. In an extremely small proportion of hospitalized patients, the initial injury is so severe that either persistent renal failure or progression to renal failure generally occurs. However, in most patients, histologic regression of the disease is the rule, and the ultimate prognosis is good. Clinical manifestations of the disease rarely recur after the first 3 months, and second episodes of acute glomerulonephritis are uncommon. Epidemic poststreptococcal acute glomerulonephritis appears to end in virtually complete resolution and healing in all patients. The prognosis is also favorable for approximately 95% of children with sporadic poststreptococcal acute glomerulonephritis. The prognosis for persons with acute glomerulonephritis secondary to other causes is less certain. The disease appears to have a poorer prognosis in adults, particularly in elderly individuals. The cause of this difference is unknown. The clinical course is largely predictable. Edema usually resolves within 5-10 days, and blood pressure (BP) usually returns to normal within 2-3 weeks, although persistence of elevated pressures for as many as 6 weeks is compatible with complete resolution. Gross hematuria usually disappears within 1-3 weeks; however, it subsequently may recur following physical activity. The C3 concentration returns to normal by 6-8 weeks after onset in more than 95% of patients. Urinary abnormalities resolve at a slower pace. Proteinuria may disappear within the first 2-3 months or may decrease slowly over 6 months. Intermittent or postural proteinuria has been noted in a few patients for as long as 1-2 years after onset. Microscopic hematuria usually disappears after 6 months; however, its presence for as long as 1 year is not uncommon. Even more prolonged hematuria (1-3 y) has been observed in some patients who ultimately have demonstrated complete resolution of their renal disease. Strongly consider the possibility that the disease has entered a chronic phase if both hematuria and proteinuria persist for more than 12 months. While clinical resolution occurs in most patients, several authors report time-related reduction in precise measurements of renal function, as well as diminished renal functional reserve. These studies further support the thesis that any significant loss of nephrons leads to hyperfiltration of the remaining units. Race No racial predilection is noted; the condition is reported in all ethnic and cultural groups. In urban populations, a predilection toward minority populations is noted; however, this may be related more to the socioeconomic factor of overcrowding than to any racial predilection. Sex The disease is more prevalent in males in all regions of the world; the male-to-female ratio range is 1.7-2:1. The reasons for this male predominance are not known. Age Acute glomerulonephritis has been reported in infants as young as 1 year and in adults as old as 90 years; however, the disease occurs with the greatest frequency in children aged 4-12 years, with a peak prevalence in individuals aged approximately 5-6 years.[2 ] Clinical History Most patients with acute glomerulonephritis (AGN) exhibit milder symptoms and/or signs somewhere between the extremes described below. o At one extreme is the asymptomatic child whose disease is discovered only by examination of the urine. Based on surveillance studies of the siblings and/or household contacts of children affected with acute poststreptococcal glomerulonephritis (APSGN), at least 50% of persons with laboratory evidence of nephritis (ie, abnormal urinalysis) appear to have no symptoms or signs of clinical illness. o At the other extreme is the child who presents with severe disease manifested by oliguria, edema, hypertension, and azotemia and with proteinuria, hematuria, and urinary casts (cylindruria). In those patients whose acute glomerulonephritis is the result of a postinfectious cause (ie, poststreptococcal acute glomerulonephritis being the most common), a latent period of 7-21 days between onset of the streptococcal infection and development of clinical glomerulonephritis is characteristic.[3 ] This latent period, more clearly defined after pharyngeal infections than after pyoderma, averages approximately 10 days. The development of clinical nephritis (ie, hematuria and/or edema) either during or within 2-5 days after the onset of a respiratory tract infection is atypical and suggests the possibility of some other form of glomerulonephritis. Gross hematuria and/or edema represent the most common clinical presentation. One or both findings usually appear abruptly and may be associated with various degrees of malaise, lethargy, anorexia, fever, abdominal pain, and headache. Observant parents may also note oliguria. An insidious onset of edema is more indicative of other forms of renal disease. An occasional child may have a scarlatiniform rash or evidence of a viral exanthema, but petechial or purpuric rashes suggest other conditions. Almost characteristic by their absence are arthralgia, arthritis, carditis, hepatic involvement, and GI bleeding. Edema is the most frequent manifesting symptom. o According to some investigators, edema is found in approximately 85% of patients. o Edema usually appears abruptly and first involves the periorbital area, but it may be generalized. o The degree of edema widely varies and depends on a number of factors, including the severity of glomerular involvement, the fluid intake, and the degree of hypoalbuminemia. Gross hematuria occurs at onset in 30-50% of children with poststreptococcal acute glomerulonephritis who require hospitalization. o The urine is usually described as being smoky, cola colored, tea colored, or rusty. The color is usually dependent on the amount of blood present and the pH of the urine. o Observant parents may note oliguria. o Clots are exceedingly rare in persons with acute glomerulonephritis. Hypertension is the third cardinal feature of poststreptococcal acute glomerulonephritis and is reported in 50-90% of children who are hospitalized with acute glomerulonephritis. The pathogenesis of the hypertension is unknown; however, pathogenesis is probably multifactorial and related only in part to extracellular fluid (ECF) volume expansion. The magnitude of the increase in blood pressure (BP) widely varies; however, systolic pressures greater than 200 mm Hg and diastolic pressures greater than 120 mm Hg are not unusual. Hypertensive encephalopathy has been reported in approximately 5% of hospitalized children and is the most serious early complication of this disease. o In these patients, hypertension is usually severe and is accompanied by signs of CNS dysfunction such as headache, vomiting, depressed sensorium, confusion, visual disturbances, aphasia, memory loss, coma, and convulsions. o Hypertensive encephalopathy has been reported in the occasional individual with minimal or no edema and with minimal urinary abnormalities. Circulatory congestion is apparent in most children admitted to the hospital but is responsible only rarely for significant early symptoms. Dyspnea, orthopnea, and cough may be present. Pulmonary rales are often audible. At times, the only evidence of congestion is detected on chest radiograph. A prominent cardiac shadow may be present until onset of diuresis. In the patient with an otherwise normal cardiovascular system, cardiac failure is unusual. Pallor is common at onset and is not explained entirely by the anemia. Physical Edema is the most frequent and sometimes the only clinical finding. Edema may be either local (eg, periorbital) or generalized. Both systolic and diastolic hypertension may be present to a varying degree. Pallor is common. In some patients, pulmonary rales are audible. Either bradycardia or tachycardia may be observed. The clinical course is largely predictable. Edema usually resolves within 5-10 days, and BP usually returns to the reference range within 2-3 weeks, although persistence of elevated pressures for 6 weeks or more is compatible with complete resolution. Gross hematuria usually disappears within 1-3 weeks; however, it may subsequently recur following physical activity. The C3 concentration returns to the reference range 6-8 weeks after onset in more than 95% of patients. Urinary abnormalities resolve at a slower pace. Proteinuria may disappear within the first 2-3 months or may slowly decrease over 6 months. Intermittent or postural proteinuria has been noted in a few patients for as long as 1-2 years after onset. Microscopic hematuria usually disappears after 6 months; however, its presence for as long as 1 year is not uncommon. Even more prolonged hematuria (1-3 y) has been observed in some patients who have ultimately demonstrated complete resolution of their renal disease. Strongly consider the possibility that the disease has entered a chronic phase if both hematuria and proteinuria persist for more than 12 months. Although clinical resolution occurs in most patients, several authors report time-related reduction in precise measurements of renal function, as well as diminished renal functional reserve. These studies further support the view that any significant loss of nephrons leads to hyperfiltration of the remaining units. A delay in the diagnosis of acute poststreptococcal glomerulonephritis is sometimes related to the absence of a clear history of a preceding documented streptococcal infections. This may be due to more stringent definition criteria that require documented evidence of past infection by streptococci. Typically, an acute nephritic syndrome develops 1-2 weeks after an antecedent streptococcal pharyngitis, whereas a lapse of 3-6 weeks is common before a nephritic syndrome develops following streptococcal pyoderma. Other factors contributing to a delay in the diagnosis of acute poststreptococcal glomerulonephritis include the sporadic occurrence of disease because this often requires a high index of suspicion, as opposed to epidemics; the history of an upper respiratory tract infection alone during the preceding month, which may not be diagnostically helpful because upper respiratory tract infections are common during winter; the misdiagnosis of severe volume overload in a child as primarily due to a cardiac cause because volume overload in children is relatively rare; and the absence of visible hematuria (a presentation that is relatively common). Physicians must consider the possibility of acute poststreptococcal glomerulonephritis in children with symptoms that may be secondary to hypertension or congestive heart failure, even in the absence of visible hematuria or a history of a preceding streptococcal infection. A urinalysis is helpful as microscopic hematuria is typically present in children with acute poststreptococcal glomerulonephritis. Causes Clinical manifestations are either the direct or indirect result of the glomerular inflammatory response, and the degree of involvement determines the severity of symptoms and signs. o Glomerular inflammation (ie, cellular proliferation, edema) reduces glomerular filtration without a coexistent decrease in total renal blood flow. o The reduced volume of glomerular filtration and the normal tubular function lead to an increase in the reabsorption of salt and water, with resulting oliguria and edema. The edema first collects in those sites where tissue resistance is low, such as the periorbital area. Later, it becomes more generalized, and, in those few patients in whom loss of albumin and/or vascular congestion is exaggerated with resultant hypoalbuminemia, the edema even may simulate that of the nephrotic syndrome. The precise etiology of the hypertension is less well explained and probably multifactorial. o ECF volume is increased, but the elevated systemic pressure does not always return to normal levels with diuresis. o o o Plasma renin levels are expected to be low due to the expanded ECF volume; however, plasma renin levels have been reported variously as low, normal, or slightly increased. The lack of response to drug-induced blockade of the renin-angiotensin system does not support this system as a primary cause for hypertension. Various cytokines known to have pressor effects are increased and may have an important role in the etiology of hypertension. Differential Diagnoses Hematuria Nephritis Other Problems to Be Considered Other postinfectious causes of acute glomerulonephritis (AGN) must be considered in the differential diagnosis (see Differentials) of acute poststreptococcal glomerulonephritis (APGN) because the syndrome of AGN has been reported following many other bacterial illnesses (eg, Streptococcus pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis, Rickettsia rickettsiae, Mycoplasma species, Meningococcus species, Leptospira species). In addition, certain viral illnesses have preceded the onset of fairly typical AGN; among the most common are varicella-zoster virus, cytomegalovirus, and the Epstein-Barr virus. In the evaluation of a patient with AGN, if evidence of a prior streptococcal infection is missing or inconclusive, then a search for another infectious cause appears appropriate. Immunoglobulin A (IgA)-associated glomerulonephritis may be confused with acute poststreptococcal glomerulonephritis.[4 ] o In the form of IgA nephropathy associated with a typical anaphylactoid purpura (ie, Henoch-Schönlein purpura [HSP] nephritis), the characteristic rash and the associated symptoms of either abdominal pain or arthritis and/or arthralgia help in the differentiation; however, in atypical cases, marked similarity may be present. o All of the clinical manifestations of acute poststreptococcal glomerulonephritis have been reported in persons with HSP nephritis, although significant hypertension and edema are found less commonly in individuals with HSP than in those with acute poststreptococcal glomerulonephritis. o Urticarial or purpuric rashes, abdominal complaints, and arthritis and/or arthralgia are found almost exclusively in persons with HSP. o Evidence of a prior streptococcal illness is usually lacking in individuals with HSP nephritis, and complement values (C3 and/or C4) are usually normal. Berger disease or IgA nephropathy usually presents as an episode of gross hematuria occurring during the early stages of a respiratory illness; no latent period occurs, and hypertension or edema is uncommon. o Recurrent episodes of gross hematuria, associated with respiratory illnesses, followed by persistent microscopic hematuria, are highly suggestive of IgA nephropathy. o In contrast, acute poststreptococcal glomerulonephritis usually does not recur, and second episodes are rare. Mesangiocapillary or membranoproliferative glomerulonephritis (MPGN) may have a presentation that is virtually identical to that of poststreptococcal acute glomerulonephritis. Distinguishing features include the following: o The initial manifestations are often more serious in persons with MPGN than in those with IgA nephropathy; the renal function is reduced markedly (ie, large elevation of serum creatinine). o Evidence of preexisting streptococcal infection is absent, although cases of MPGN have been reported in which clear evidence of such an infection is present. o In most cases, C3 levels are depressed persistently, longer than 6 weeks. o Urinary abnormalities persist past the time of expected resolution for acute poststreptococcal glomerulonephritis. Crescentic glomerulonephritis is the term used to describe a histologic picture of severe proliferative glomerulonephritis. o In persons with crescentic glomerulonephritis, in addition to inflammatory changes within the glomerular tuft, extensive proliferation exists within the Bowman space, leading to the formation of synechiae between the glomerular tuft and Bowman capsule. o The clinical picture is generally referred to as rapidly progressive glomerulonephritis and may be secondary to numerous causes, including an immune-complex mediated poststreptococcal nephritis. The initial clinical picture is generally severe, and resolution appears delayed. Other forms of glomerulonephritis (eg, systemic lupus erythematosus [SLE] nephritis, familial nephritis, chronic glomerulonephritis) may occasionally be confused with acute poststreptococcal glomerulonephritis when an acute exacerbation of the previously present nephropathy is present. In addition to the lack of expected complete resolution, other features suggest a condition other than acute poststreptococcal glomerulonephritis. The differential diagnosis of acute glomerulonephritis can be divided as follows (percentages indicate approximate frequency of C3 or hemolytic complement):[5 ] Low serum complement level o Systemic diseases SLE (focal, 75%; diffuse, 90%) Subacute bacterial endocarditis (90%) Visceral abscess "Shunt" nephritis (90%) Cryoglobulinemia (58%) o Renal diseases Acute postinfectious glomerulonephritis (>90%) MPGN - Type I (50-80%), type 2 (80-90%) Normal serum complement level o Systemic diseases Polyarteritis nodosa group Hypersensitivity vasculitis o Wegener granulomatosis HSP Goodpasture syndrome Renal diseases IgA (or IgG-IgA) nephropathy Idiopathic rapidly progressive glomerulonephritis (RPGN) Anti-glomerular basement membrane (GBM) disease Negative immunofluorescence findings Immune complex disease Workup Laboratory Studies Urine o Urine output most is often reduced in acute glomerulonephritis (AGN), and urine is concentrated and acidic. o Glucosuria occurs occasionally, and proteinuria is commonly present. o The urine reaction for protein rarely exceeds 3+ by dipstick, corresponding to fewer than 2 g/m2/d when assessed quantitatively. o Approximately 2-5% of children with acute poststreptococcal glomerulonephritis (APSGN) have massive proteinuria and a nephrotic picture. o Hematuria is the most consistent urinary abnormality, although histologic findings consistent with poststreptococcal acute glomerulonephritis have been reported in children who had no or minimal urinary abnormalities. o Polymorphonuclear leukocytes and renal epithelial cells are common in the urine of patients with poststreptococcal acute glomerulonephritis, particularly during the early phase of the disease. o Hyaline and/or cellular casts are almost always present. o RBC casts have been found in 60-85% of hospitalized children with poststreptococcal acute glomerulonephritis. RBC casts, although characteristic of a glomerular lesion, are often not detected because the urine is not fresh or is examined by an inexperienced person. Streptococcal infection o Look for evidence of streptococcal infection in all patients. o Cultures from either the pharynx or skin may be positive; however, high streptococcal antibody titers are more compelling. o Numerous laboratory tests can be used to measure antibodies to various streptococcal antigens (eg, ASO, AH, anti-DNase B) or to combinations of antigens (eg, streptozyme test). o Whatever test is used, a rise in the titer of the antibody, measured at an interval of 2-3 weeks, is more meaningful than a single measurement. An ASO titer of 250 U or higher is highly suggestive of recent streptococcal infection. Hemolytic complement o Total hemolytic complement and some of its components are low during poststreptococcal acute glomerulonephritis. o o o o o o Renal o o o o The concentration of C3 has been found to be decreased in more than 90% of patients with poststreptococcal acute glomerulonephritis when measured serially during the first 2 weeks of the illness. Total hemolytic complement values are also depressed. These tests help to differentiate poststreptococcal from other postinfectious forms of acute glomerulonephritis. C4 levels are most often normal. Serum levels of fifth component of complement (C5) and properdin are usually decreased. The complement levels generally return to normal by 6-8 weeks after onset. The extent of renal functional impairment is correlated directly to the severity of the glomerular injury. The elevation in the serum concentrations of creatinine and BUN is usually modest, although some patients may have severe azotemia at onset. The electrolyte profile is usually normal; hyperkalemia and metabolic acidosis are only present in patients with significant renal functional impairment. The same applies to hyperphosphatemia. Total serum calcium, but not ionized calcium levels, may be low in patients who have a nephrotic picture. Blood o o o o A mild anemia (normocytic, normochromic) is common in persons in the early phase of acute glomerulonephritis; its degree tends to parallel the degree of extracellular (ECF) volume expansion. Erythropoiesis may decline in the aftermath of acute glomerulonephritis, particularly in individuals with severe cases. WBC and platelet counts are usually normal, although an occasional patient exhibits a leukocytosis; rarely, a mild thrombocytopenia may be present. A few patients have hypoproteinemia and hyperlipidemia. A nephrotic picture has been reported in approximately 5% of hospitalized patients with poststreptococcal acute glomerulonephritis. Imaging Studies No specific radiologic studies are particularly helpful in either the evaluation or the treatment of patients with acute glomerulonephritis. o Renal ultrasonography generally demonstrates normal to slightly enlarged kidneys bilaterally with some evidence of increased echogenicity. o Chest radiographs commonly demonstrate central venous congestion in a hilar pattern, the degree of which parallels the increase in ECF volume. Occasionally, an enlarged cardiac shadow is evident. Other Tests Currently, no other tests provide any meaningful data regarding acute glomerulonephritis. Procedures No procedures are recommended routinely in the evaluation of patients with acute glomerulonephritis. The performance of a renal biopsy is indicated in patients whose clinical presentation, laboratory findings, or course is atypical. In such persons, study of the histology by light, immunofluorescent, and electron microscopy may be diagnostic. Histologic Findings Histologic findings depend on the etiology of the acute glomerulonephritis, the severity of the inflammatory process, and the stage of the disease at the time of the biopsy. The section below concentrates on the findings observed in individuals with poststreptococcal acute glomerulonephritis. The severity of the histologic process correlates with the clinical severity of the initial phase of the disease, and it may correlate with the ultimate prognosis (ie, severe lesions have worse prognoses). By light microscopy, the glomerular tufts appear enlarged and swollen, often filling Bowman space. A moderate-to-marked increase in proliferation of both mesangial and epithelial cells is present. Polymorphonuclear leukocytes often are observed, and monocytes also may be present. The increased cell mass expands the central lobular area in a centrifugal pattern, leading to narrowing of the capillary lumens. When the inflammatory process is extensive, the epithelial cells of Bowman capsule proliferate, forming crescents. Few, if any, tubular changes are noted. Granular deposits of immunoglobulin G (IgG) and C3 along the capillary walls generally are observed when the specimen is studied by immunofluorescent microscopy early in the course of the disease. Other immunoglobulins (eg, immunoglobulin M [IgM]), complement components (eg, C4), properdin, and fibrinogen often are observed. Later in the course of the disease, the immunoreactants are observed primarily in the mesangium. In the nonstreptococcal forms of postinfectious glomerulonephritis, no significant deposition of complement components is present, although either IgG or IgM may be observed (as may immunoglobulin A [IgA] in persons with Henoch-Schönlein purpura (HSP) or IgA nephropathy). Electron microscopy of renal tissue from patients with poststreptococcal acute glomerulonephritis usually reveals electron-dense deposits (humps) in the subepithelial space. During the recovery process, these deposits rapidly disappear, although fragments still may be found in the mesangium. Renal biopsy is not needed in most patients with poststreptococcal acute glomerulonephritis (see Procedures). Treatment Medical Care By the time the child with acute poststreptococcal glomerulonephritis (APSGN) presents with symptoms, the glomerular injury has already occurred, and the healing process has begun. Thus, influencing the ultimate course of the disease by any specific therapy directed at the cause of the nephritis is not possible. Conversely, morbidity and early mortality are influenced considerably by appropriate medical therapy. Even then, treatment is usually supportive and directed toward the potential complications. General management begins with a decision to admit the child with acute glomerulonephritis (AGN) to the hospital or merely have him or her undergo frequent outpatient examinations. Hospitalization is indicated if the child has significant hypertension or a combination of oliguria, generalized edema, and elevation of serum creatinine or potassium. Severe hypertension, or that associated with signs of cerebral dysfunction, demands immediate attention. o Debate exists regarding the agent that is most effective in patients with severe hypertension. Three drugs are commonly cited as having a high benefit-to-risk ratio: labetalol (0.5-2 mg/kg/h intravenously [IV]), diazoxide, and nitroprusside (0.5-2 mcg/kg/min IV, in patients with severe hypertension that is refractory to the previous agents); with any of these agents, the simultaneous IV administration of furosemide at doses of 2 mg/kg may be merited. Diazoxide use for blood pressure (BP) control is limited because, once administered, no further control of pressure is possible, unlike labetalol or nitroprusside. o Severe hypertension without encephalopathy can be treated in the manner just described or, more commonly, by administration of vasodilator drugs such as hydralazine or nifedipine. The doses of these drugs can be administered either by injection or by mouth and can be repeated every 10-20 minutes until a suitable response is obtained. For most children, the need for more than 2-3 doses is unusual. Mild-to-moderate hypertension does not warrant emergency management. o Mild-to-moderate hypertension is treated most effectively with bedrest, fluid restriction, and less-frequent doses of the preceding medications. o The use of loop diuretics, such as furosemide (1-3 mg/kg/d oral [PO], administered 1-2 times daily), may hasten resolution of the hypertension. o For patients resistant to treatment, either hydralazine or nifedipine is indicated. o ACE inhibitors are effective, although they have the potential to produce hyperkalemia and usually are not first-line drugs in acute glomerulonephritis. Edema and circulatory congestion are usually not sufficiently marked to produce more than minimal discomfort. o Restriction of fluids to those amounts needed to replace insensible losses is the best treatment for edema and circulatory congestion. o Loop diuretics (furosemide) administered PO have been reported to reduce the length of hospitalization in children who are edematous. o If congestion is marked, administer furosemide parenterally (2 mg/kg). o Phlebotomy, rotating tourniquets, dialysis, or digitalization is rarely necessary. Anuria or severe and persistent oliguria may occur in 3-6% of children with acute glomerulonephritis and may necessitate hospitalization. o Fortunately, anuria or severe and persistent oliguria is usually transient. o Because they may be ototoxic, avoid large doses of furosemide in children with these symptoms. o Osmotic diuretics, such as mannitol, are contraindicated as they might increase vascular volume. A course of penicillin can be administered to avoid contamination of contacts with a nephritogenic strain of streptococci; however, in most instances, these contacts do not develop overt acute glomerulonephritis. Such therapy does not influence the course of the disease in the index patient, but it may alter the response that confers type-specific immunity. Throat cultures of immediate family members might detect patients who are asymptomatic but infected. Steroid therapy is indicated only in patients with severe crescentic glomerulonephritis or in those with rapidly progressive glomerulonephritis. o Selected patients with Henoch-Schönlein purpura (HSP) nephritis and membranoproliferative glomerulonephritis (MPGN) also may benefit from such agents. o An experienced nephrologist should make decisions regarding the indication for such treatment. Surgical Care If indicated at any time during the course of the disease, an experienced nephrologist should perform renal biopsy percutaneously. Consultations The general pediatrician should be capable of treating patients with mild-to-moderate acute glomerulonephritis. Consultation with a pediatric nephrologist is necessary when one or more of the following are present: Severe hypertension Severe oliguria Severe edema Nephrotic-range proteinuria Azotemia (moderate to marked) Recurrent episodes of gross hematuria Persistently depressed C3 (past 8-10 wk) Atypical onset o Absence of latent period o No evidence of streptococcal illness Failure of expected resolution of clinical signs o Gross hematuria within the preceding 10-14 days o o o o o Microscopic hematuria within 1 year Edema within 2 weeks Proteinuria (>50 mg/dL) within 6 months Azotemia within 1 week Hypertension within 6 weeks Diet A low-sodium, low-protein diet should be prescribed during the acute phase, when edema and hypertension are in evidence; however, prolonged dietary restrictions are not warranted. Limitation of fluid and salt intake is recommended in the child who has either oliguria or edema. Curtailment of fluid to amounts consistent with insensible losses helps to minimize vascular overload and hypertension. Activity Limited activity is probably indicated during the early phase of the disease, particularly if hypertension is present. Bedrest may lessen the degree and duration of gross hematuria if present; however, longer periods of bedrest do not appear to influence the course or long-term prognosis; therefore, they are generally not recommended. Medication Need for medicines are usually limited in scope and in length. Administer antibiotics (penicillin or erythromycin) for 10 days to ensure eradication of the streptococcus if the disease is believed to be acute poststreptococcal glomerulonephritis (APSGN) and if risk of contamination is present. Some clinicians use this treatment only when evidence suggests an active infection. Antihypertensives (see Medical Care) usually are not necessary after the child leaves the hospital, although mild hypertension may persist for as many as 6 weeks. The medications that can be used span the entire range of antihypertensives such as vasodilators (eg, hydralazine), calcium channel-blocking agents (eg, long-acting nifedipine, amlodipine), or ACE inhibitor (eg, enalapril). Carefully monitor blood pressure (BP) for at least 1 week after the drug is discontinued to ensure that rebound hypertension does not occur. Diuretic agents (eg, furosemide) are rarely necessary after the first 2 days; hypertension persisting beyond the first week may suggest a diagnosis other that acute glomerulonephritis. Antihypertensives These agents are commonly used during initial phase. Indications for use are covered in appropriate sections. Specific agents are mentioned below. Amlodipine (Norvasc) Generally regarded as a dihydropyridine, although experimental evidence suggests that it may also bind to the nondihydropyridine binding sites. Appropriate for the prophylaxis of variant angina. Elicits antianginal and antihypertensive effects. Blocks postexcitation release of calcium ions into cardiac and vascular smooth muscle, thereby inhibiting the activation of ATPase on myofibril contraction. The overall effect is reduced intracellular calcium levels in cardiac and smooth muscle cells of the coronary and peripheral vasculature, resulting in dilatation of coronary and peripheral arteries. Also increases myocardial oxygen delivery in patients with vasospastic angina. May also potentiate ACE inhibitor effects. During depolarization, inhibits calcium ions from entering slow channels and voltage-sensitive areas of vascular smooth muscle and myocardium. Benefits nonpregnant patients with systolic dysfunction, hypertension, or arrhythmias. Has a substantially longer half-life than nifedipine and diltiazem and is administered qd. Dosing Adult 5 mg PO qd initially, decrease to 2.5 mg PO qd if elderly or secondary agent; may increase after 1-2 wk; not to exceed 10 mg/d Pediatric <6 years: Not established 6-17 years: 2.5-5 mg PO qd Interactions Fentanyl and alcohol may increase hypotensive effects; calcium channel blockers may increase cyclosporine levels; H2 blockers (cimetidine), erythromycin, nafcillin, and azole antifungals may increase toxicity (avoid combination or monitor closely); carbamazepine may reduce bioavailability (avoid this combination); rifampin may decrease levels (monitor and adjust dose of calcium channel blocker) Contraindications Documented hypersensitivity Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Adjust dose in renal/hepatic impairment; may cause lower extremity edema; allergic hepatitis have occurred but is rare Diazoxide (Hyperstat) Diuretic benzothiazine antihypertensive agent that may be indicated for emergency reduction of severe hypertension. Only administer IV. Produces direct smooth muscle relaxation of peripheral arterioles, which decreases BP. Dosing Adult 1-3 mg/kg IV as single injection, not to exceed 150 mg/dose; repeat dose in 5-15 min prn until BP is reduced adequately Pediatric 2-3 mg/kg IV administered over 30 min; may repeat in 30-60 min Interactions May decrease serum hydantoins, possibly resulting in decreased anticonvulsant effects; thiazide diuretics may potentiate hyperuricemic and antihypertensive effects Contraindications Documented hypersensitivity; aortic coarctation; pheochromocytoma; arteriovenous shunts; aortic aneurysm; significant hypoproteinemia (serum albumin <2.5 g/dL) Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Carefully monitor BP while administering medication and for succeeding few hours; hypotension is relatively common adverse effect; may produce hyperglycemia with administration of multiple doses; patients with diabetes mellitus may require insulin; when administered prior to delivery, may produce fetal or neonatal hyperbilirubinemia, thrombocytopenia, altered carbohydrate metabolism, and other adverse reactions Furosemide (Lasix) Loop diuretic useful in patients with AGN who are edematous. Also has some BP-lowering effect. In acute hypertensive states, administer IV furosemide. Increases excretion of salt and water by interfering with chloride-binding cotransport system in ascending loop of Henle. Dosing Adult 20-80 mg/d PO/IV/IM; titrate not to exceed 600 mg/d for severe edematous states Pediatric 1-2 mg/kg/dose PO, not to exceed 6 mg/kg/dose; do not administer more frequently than q6h; 1 mg/kg IV/IM slowly under close supervision, not to exceed 6 mg/kg Interactions Metformin decreases concentrations; interferes with hypoglycemic effect of antidiabetic agents and antagonizes muscle-relaxing effect of tubocurarine; auditory toxicity appears to be increased with coadministration of aminoglycosides; hearing loss of varying degrees may occur; anticoagulant activity of warfarin may be enhanced when taken concurrently; increased plasma lithium levels and toxicity are possible when taken concurrently Contraindications Documented hypersensitivity; hepatic coma; anuria; state of severe electrolyte depletion Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Perform serum electrolyte, carbon dioxide, glucose, creatinine, uric acid, calcium, and BUN measurements Labetalol (Normodyne, Trandate) Blocks beta1-adrenergic, alpha-adrenergic, and beta2-adrenergic receptor sites, decreasing BP. Dosing Adult 0.5-3 mg/kg/h IV; gradually titrate dose according to BP response Pediatric Not established; limited data suggest 0.4-1 mg/kg/h IV; not to exceed 3 mg/kg/h Interactions Decreases effect of diuretics and increases toxicity of methotrexate, lithium, and salicylates; may diminish reflex tachycardia, resulting from nitroglycerin use, without interfering with hypotensive effects; cimetidine may increase labetalol blood levels; glutethimide may decrease labetalol effects by inducing microsomal enzymes Contraindications Documented hypersensitivity; cardiogenic shock; pulmonary edema; bradycardia; atrioventricular block; uncompensated congestive heart failure; reactive airway disease; severe bradycardia; asthma and/or obstructive airway disease Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Caution in impaired hepatic function; discontinue therapy with signs of liver dysfunction; in elderly patients, a lower response rate and higher incidence of toxicity may be observed Hydralazine (Apresoline) Decreases systemic resistance through direct vasodilation of arterioles. Dosing Adult Acute hypertension: 0.15-0.3 mg/kg/dose PO/IM q10-20 min Chronic hypertension: 0.75-5 mg/kg/d PO divided q6-12h; gradually titrate over 3-4 weeks; not to exceed 200 mg/d Pediatric Administer as in adults Interactions MAOIs and beta-blockers may increase toxicity; pharmacologic effects may be decreased by indomethacin Contraindications Documented hypersensitivity; mitral valve rheumatic heart disease Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Has been implicated in myocardial infarction; caution required in suspected coronary artery disease Nifedipine (Adalat, Procardia) Relaxes coronary smooth muscle and produces coronary vasodilation, which, in turn, improves myocardial oxygen delivery. Dosing Adult 10-30 mg IR cap PO tid, not to exceed 120-180 mg/d 30-60 mg SR tab PO qd, not to exceed 90-120 mg/d Pediatric Acute hypertension: 0.05-0.1 mg/kg/dose PO/SL q10-20min Chronic hypertension: 0.25-0.5 mg/kg/dose PO tid/qid prn Interactions Caution with coadministration of any agent that can lower BP, including beta-blockers and opioids; H2 blockers (cimetidine) may increase toxicity Contraindications Documented hypersensitivity Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions May cause lower extremity edema; allergic hepatitis has occurred but is rare Nitroprusside (Nitropress) Produces vasodilation and increases inotropic activity of heart. Higher dosages may exacerbate myocardial ischemia by increasing heart rate. Dosing Adult Begin infusion at 0.3-0.5 mcg/kg/min IV and titrate upward by increments of 0.5 mcg/kg/min; titrate to desired effect; average dose is 1-6 mcg/kg/min Infusion rates >10 mcg/kg/min may lead to cyanide toxicity Pediatric Administer as in adults Interactions None reported Contraindications Documented hypersensitivity; subaortic stenosis; idiopathic hypertrophic and atrial fibrillation or flutter Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Caution in increased intracranial pressure, hepatic failure, severe renal impairment, and hypothyroidism; in renal or hepatic insufficiency, nitroprusside levels may increase and can cause cyanide toxicity; sodium nitroprusside has the ability to lower BP, thus use only in those patients with mean arterial pressures >70 mm Hg Antibiotics Administer antibiotics for 10 d to ensure eradication of streptococci, if the disease is believed to be poststreptococcal acute glomerulonephritis and risk of contamination is present. Penicillin (Beepen-VK, Betapen-VK, Pen-Vee K, V-Cillin K) Inhibits biosynthesis of cell wall mucopeptide. Bactericidal against sensitive organisms when adequate concentrations are reached; most effective during stage of active multiplication. Dosing Adult 250-500 mg PO q6-8h for 10 d Pediatric <12 years: 25-50 mg/kg/d PO divided tid/qid, not to exceed 3 g/d for 10 d >12 years: Administer as in adults Interactions Probenecid may increase effectiveness by decreasing clearance; tetracyclines are bacteriostatic, causing decrease in effectiveness of penicillins when administered concurrently Contraindications Documented hypersensitivity Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Caution in renal impairment Erythromycin (E.E.S., E-Mycin, Eryc, Ery-Tab, Erythrocin) Inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNA-dependent protein synthesis to arrest. Dosing Adult 250 mg erythromycin stearate and/or base (or 400 mg ethylsuccinate) PO q6h 1 h ac, or 500 mg q12h Alternatively, 333 mg q8h for 10 d Pediatric 30-50 mg/kg/d (15-25 mg/lb/d) PO divided q6-8h for 10 d Interactions Coadministration may increase toxicity of theophylline, digoxin, carbamazepine, and cyclosporine; may potentiate anticoagulant effects of warfarin; coadministration with lovastatin and simvastatin increases risk of rhabdomyolysis Contraindications Documented hypersensitivity; hepatic impairment Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Caution in liver disease; estolate formulation may cause cholestatic jaundice; GI adverse effects are common (administer doses pc); discontinue use if nausea, vomiting, malaise, abdominal colic, or fever occurs Follow-up Further Inpatient Care Only a small percentage of patients with acute glomerulonephritis (AGN) require initial hospitalization, and most of those are ready for discharge in 2-4 days. As soon as the blood pressure (BP) is under relatively good control and diuresis has begun, most children can be discharged and monitored as outpatients. Further Outpatient Care Follow up at 0-6 weeks as frequently as necessary to determine the following: o Hypertension has been controlled. o Edema has started to resolve. o Gross hematuria has resolved. o Azotemia has resolved. Follow up 8-10 weeks after onset to determine the following: o Azotemia has subsided. o Anemia has been corrected. o Hypertension has resolved. o C3 and C4 concentrations have returned to normal. Follow up at 3, 6, and 9 months after onset to check the following: o Hematuria and proteinuria are subsiding gradually. o BP is normal. Follow up at 12 months after onset to determine the following: o Proteinuria has disappeared. o Microscopic hematuria has disappeared. Follow up at 2, 5, and 10 years after onset to check the following: o Urine is normal. o BP is normal. o Serum creatinine level is normal. Inpatient & Outpatient Medications See Medical Care. Transfer Transfer of responsibility for the patient with AGN rarely indicated except in those instances in which a consultation with a nephrologist is not easily obtainable in the local area or by telephone, facsimile, or e-mail. See Consultations for instances that necessitate consultation with a pediatric nephrologist. Complications Acute complications as part of the initial syndrome are discussed in History. The most common acute complication is hypertension with or without CNS manifestations. Anemia is common early and is primarily due to dilution. Anemia tends to resolve with diuresis. A few patients may have diminished erythropoiesis in the recovery phase and have some persisting anemia. An occasional patient develops pulmonary edema because of the marked increase in vascular volume that is present in the early phase of the disease. Congestive heart failure is rare but has been reported. Definite myocarditis has also been documented. Azotemia is discussed in History, but its persistence or worsening is always troubling and may suggest acute renal failure (ARF). ARF may suggest an alternate diagnosis (eg, membranoproliferative glomerulonephritis [MPGN], Henoch-Schönlein purpura [HSP], systemic lupus erythematosus [SLE]) or a severe or worsening acute poststreptococcal glomerulonephritis, such as observed in those with crescentic glomerulonephritis or rapidly progressive glomerulonephritis. Prognosis Epidemic poststreptococcal acute glomerulonephritis appears to end in virtually complete resolution and healing in all patients, and the prognosis is favorable for most children with acute sporadic poststreptococcal glomerulonephritis. o Edema usually resolves within 5-10 days, and BP usually returns to normal after 2-3 weeks, even though persistence of elevated pressures for as many as 6 weeks is compatible with complete resolution. o Gross hematuria usually disappears within 1-3 weeks but may be exacerbated by physical activity. o C3 concentration returns to normal in more than 95% of patients by the end of 810 weeks. Urinary abnormalities resolve at various times after onset. o Proteinuria may disappear within the first 2-3 months or may slowly decrease over 6 months. Intermittent or postural proteinuria has been noted for 1-2 years after onset. o Microscopic hematuria usually disappears after 6 months, but its presence for as long as 1 year should not cause undue concern, and even more prolonged hematuria (1-3 y) has been observed. o Consider the possibility of chronic renal disease when both hematuria and proteinuria persist longer than 12 months. The ultimate prognosis in individuals with acute poststreptococcal glomerulonephritis largely depends on the severity of the initial insult. o In a few hospitalized patients, the initial injury is so severe that either persistent renal failure or progressive renal failure ensues. o However, histologic regression of the disease in most patients is predictable, and the ultimate prognosis is good. o Clinical manifestations of the disease rarely recur after the first 3 months, and second episodes of acute glomerulonephritis are rare. Patient Education Clearly and specifically explain the nature of the disease, its course, and the eventual prognosis of the condition to the child (if old enough to understand) and the parents and/or caregivers. They need to understand that, while complete resolution is expected, a small possibility exists for persistent disease, and that an even smaller possibility exists for progression. This information is necessary for some patients to ensure that compliance with the follow-up program occurs. Clearly outline a follow-up plan and discuss the plan with the family. BP measurements and urine examinations for protein and blood constitute the basis of the follow-up plan. Perform examinations at 4-week to 6-week intervals for the first 6 months and at 3-month to 6-month intervals thereafter until both hematuria and proteinuria have been absent and BP has been normal for 1 year. Documenting that the low C3 has returned to normal after 8-10 weeks may be useful. For excellent patient education resources, visit eMedicine's Kidneys and Urinary System Center. Also, see eMedicine's patient education article Blood in the Urine. Miscellaneous Medicolegal Pitfalls Failure to perform a thorough and complete history Failure to perform a complete physical examination Failure to inform the parents and/or child of the working diagnosis Failure to pay attention to the troublesome problems of hypertension, edema, and azotemia Failure to reassure the parents and/or child at intervals Failure to perform only the necessary examinations and discuss the results with the family Failure to obtain consultations as needed with an appropriate explanation of the purpose of the consultation Failure to develop a follow-up plan Failure to keep the family informed of the child's progress and how this progress relates to the diagnosis, management, and prognosis Special Concerns No special concerns are pertinent other than those noted in the History and Medical Care sections. Emphasizing other situations is also worthwhile. Diagnosis: The course and prognosis for acute poststreptococcal glomerulonephritis (APSGN) well known and almost always favorable. This is not so with nonstreptococcal forms of postinfectious glomerulonephritis. For that reason, attempting to identify the etiology of the condition is prudent. Complement profiles in poststreptococcal acute glomerulonephritis: Theoretically, the complement (or C3) levels should be decreased in all such patients; however, the duration of low values may be quite brief and, therefore, missed, even when examined serially. When the serum level is low in individuals with acute poststreptococcal glomerulonephritis, a depressed level for longer than 6-8 weeks is unusual. Thus, if the value remains low after this period of time, thinking of some other nephritic process, such as membranoproliferative glomerulonephritis (MPGN), is wise. Renal biopsy is not usually indicated in the person who presents with relatively typical acute poststreptococcal glomerulonephritis. However, in individuals whose initial presentation or early course is atypical, an examination of renal tissue may be indicated. Hypertensive encephalopathy can be the presenting feature of postinfectious glomerulonephritis and is the most serious of the acute complications of this disease. Because the urinalysis in such patients may exhibit minimal abnormalities, the underlying cause may not be readily apparent. A high index of suspicion is required to make an appropriate diagnosis. The prognosis for individuals with acute poststreptococcal glomerulonephritis is not as good for adults as it appears to be for children. Prolonged follow-up observation appears to be indicated. eMedicine Specialties > Pediatrics: General Medicine > Infectious Disease Streptococcal Infection, Group A Mark R Schleiss, MD, American Legion Chair of Pediatrics, Professor of Pediatrics, Division Director, Division of Infectious Diseases and Immunology, Department of Pediatrics, University of Minnesota Medical School Updated: Nov 20, 2009 Introduction Background Streptococcus pyogenes (group A Streptococcus) is one of the most important pathogens encountered in clinical practice. An understanding of the diverse nature of infectious disease complications attributable to this organism is an important cornerstone of pediatric medicine. In addition to infections of the upper respiratory tract and the skin, S pyogenes can cause a wide variety of invasive systemic infections, and infection with this pathogen is also causally linked to 2 potentially serious nonsuppurative complications: acute rheumatic fever and acute glomerulonephritis. Recently, infection with S pyogenes has reemerged as an important cause of toxic shock syndrome (TSS), as well as life-threatening skin and soft tissue infections, especially necrotizing fasciitis. Clinical syndromes compatible with S pyogenes infection have been documented in humans for many centuries. S pyogenes was likely responsible for the apparent scarlet fever epidemic described by Hippocrates in the fifth century BC. The first modern description of streptococcal infection was based on the demonstration of the organism in cases of erysipelas and wound infection by Billroth in 1874. In 1884, Pasteur was the first to report isolation of this organism from the bloodstream in a woman with puerperal sepsis. The organism was designated S pyogenes by Rosenbach in the late 19th century. Another important historical milestone was the description of the classic differential patterns of alpha, beta, and gamma hemolysis on blood agar plates, which was described by Brown in 1919. This observation allowed differentiation of pathogenic streptococci. Perhaps the major historic turning point in the classification of streptococcal infections occurred in the early 1930s by Rebecca Lancefield with her pioneering work in the identification and description of distinct streptococcal serogroups. The work of Lancefield was instrumental in leading to the important classification of beta-hemolytic strains into distinct serogroups. This insight, in turn, led to the recognition that serogroup A isolates (S pyogenes) were the streptococcal strains responsible for pharyngitis, pyoderma, and nonsuppurative sequelae. Pathophysiology Description and identification of the organism Streptococci are gram-positive cocci that tend to grow as pairs and short chains in clinical specimens. When cultured on blood agar plates, the production of a characteristic zone of complete hemolysis (beta-hemolysis) is an important clue to classification. S pyogenes produces beta-hemolysis, in contrast to the zone of partial hemolysis (alpha-hemolysis) generated by Streptococcus pneumoniae. As originally described by Lancefield, beta-hemolytic streptococci can be divided into many groups based on the antigenic differences in group-specific polysaccharides located in the bacterial cell wall. More than 20 serologic groups have been identified and designated by letters (eg, A, B, C). Of the non–group A streptococci, the group B strain is the most important human pathogen (the most common cause of neonatal sepsis and bacteremia), although other groups (particularly group G) have occasionally been implicated as causes of pharyngitis. Although serologic grouping by the Lancefield method is the criterion standard for differentiation of pathogenic streptococcal species, group A organisms can be identified more cost effectively by numerous latex agglutination, coagglutination, or enzyme immunoassay procedures. Group A strains can also be distinguished from other groups by their sensitivity to bacitracin. A disc that contains 0.04 U of bacitracin inhibits the growth of more than 95% of group A strains, whereas 80-90% of non–group A strains are resistant to this antibiotic. The bacitracin disc test is simple to perform and interpret in an office-based laboratory and is sufficiently accurate for presumptive identification of group A streptococci. Presumptive identification of a strain as group A streptococci can also be made on the basis of production of the enzyme L-pyrrolidonyl-beta-naphthylamide (PYRase). Among the betahemolytic streptococci isolated from throat culture, only group A isolates produce PYRase, which can be identified on the basis of the characteristic color change (red) after inoculation of a disk on an agar plate followed by overnight incubation. Cellular constituents and virulence factors The somatic cellular constituents as well as the extracellular enzymes and toxins of S pyogenes are responsible for many of the pathogenic effects observed in vivo. These are summarized as follows: Intrinsic (somatic) constituents o M protein o Hyaluronic acid o Lipoteichoic acid o Protein F o Serum opacity factor (OF) o T protein Extracellular streptococcal proteins o Streptococcal pyogenic exotoxins (SPEs) o Streptolysin O o Streptolysin S o Deoxyribonucleases (DNAses) o Hyaluronidase o Streptokinase o Nicotinamide adenine dinucleotidase (NADase) The major virulence factor of the organism is the M protein. This protein, a stable dimer, is anchored to the cell membrane and traverses and penetrates the cell wall. The proximal portion of the molecule is highly conserved among group A isolates, whereas the distal portions contain type-specific epitopes localized on the tips of fibrils (fimbriae) that protrude from the cell surface. The ability of group A streptococci to initiate disease is highly depends on M protein.[1 ] Strains lacking M protein are essentially nonpathogenic. Interestingly, streptococci isolated from chronic pharyngeal carriers (individuals asymptomatically colonized with S pyogenes) contain little or no M protein and are also relatively avirulent. Molecular mechanisms by which M protein mediates pathogenesis are complex. In the nonimmune host, M protein mediates an antiphagocytic effect by inhibition of activation of the alternate complement pathway. Acquired immunity to streptococcal infection is based on the development of opsonic antibodies directed against the antiphagocytic epitopes of M protein. Although such antibodies protect from infection against a homologous M protein type, they unfortunately confer no immunity against other M types. This observation is one of the factors that represent a major theoretical obstacle to the S pyogenes vaccine design because more than 80 M serotypes have been described to date. Community-based outbreaks of particular streptococcal diseases tend to be associated with certain M types; therefore, M serotyping has been very valuable for epidemiologic studies. Other streptococcal cell wall antigens are important in pathogenesis and epidemiologic typing of S pyogenes. Most strains are enveloped in a hyaluronic acid capsule that serves as an accessory virulence factor by inhibiting phagocytosis. Lipoteichoic acid and protein F are cell wall constituents that play roles in the adherence of S pyogenes to fibronectin on the surface of human epithelial cells, an important event in the initiation of the infectious process. Serum OF is a lipoproteinase associated with M protein that serves in classifying strains not identifiable by M typing. Another streptococcal protein, T protein, does not appear to be a virulence factor but shows significant antigenic variation among clinical isolates. Therefore, T typing is a useful adjunct to M typing for epidemiologic studies of group A streptococcal outbreaks. Another typing schemes that has been used to characterize and measure the genetic diversity among isolates of S pyogenes is emm typing, which is based on sequence at the 5' end of a locus (emm) that is present in all isolates. The targeted region of emm displays the highest level of sequence polymorphism known for a S pyogenes gene; more than 150 emm types have been described to date.[2 ]The emm gene encodes the M protein, which forms the basis of a serological typing scheme described above. There are 4 major subfamilies of emm genes, which are defined by sequence differences within the 3' end, encoding the peptidoglycan-spanning domain. The chromosomal arrangement of emm subfamily genes reveals 5 major emm patterns, designated as emm patterns A through E. An example of the usefulness of emm typing is described by McGregor et al.[3 ] In addition to somatic constituents, group A streptococci produce a wide variety of extracellular enzymes and toxins important in pathogenesis. The family of SPEs includes SPEs A, B, C, and F. These toxins are responsible for the rash of scarlet fever. These toxins are further responsible for other pathogenic effects on the host, including pyrogenicity, cytotoxicity, and enhancement of susceptibility to endotoxin. SPE B is a precursor for a cysteine protease, another determinant of virulence. Group A streptococcal isolates associated with streptococcal TSS encode certain SPEs (ie, A, C, F) capable of functioning as superantigens. These antigens induce a marked febrile response, induce proliferation of T lymphocytes, and induce synthesis and release of multiple cytokines, including tumor necrosis factor, interleukin-1 beta, and interleukin-6. This activity is attributed to the ability of the superantigen to simultaneously bind to the V-beta region of the T-cell receptor and to class II major histocompatibility antigens of antigen-presenting mononuclear cells, resulting in widespread nonspecific T-cell proliferation and increased production of interleukin-2. S pyogenes also elaborates 2 distinct hemolysins. These proteins are responsible for the zone of hemolysis observed on blood agar plates and are also important in the pathogenesis of tissue damage in the infected host. Streptolysin O is toxic to a wide variety of cell types, including myocardium. Streptolysin O is highly immunogenic, and determination of the antibody responses engendered to this protein (ASO titer) is often useful in the serodiagnosis of recent infection. Streptolysin S is another virulence factor capable of damaging polymorphonuclear leukocytes and subcellular organelles; however, in contrast to streptolysin O, it does not appear to be immunogenic. Other extracellular products are elaborated by S pyogenes that may play a role in tissue damage and spread of organisms through tissue planes. These products include a family of DNAses (ie, DNAses A-D), hyaluronidase, and streptokinase. Other virulence factors of streptococci include NADase, proteinase, C5a-peptidase, amylase, and esterase, although the role of these proteins in pathogenesis is less well understood. A characteristic of S pyogenes studied in greater detail is the ability of the organism to invade epithelial cells. Failure of penicillin to eradicate S pyogenes from the throats of patients, especially those who are carriers of S pyogenes, has been increasingly reported. The viability of ingested, intracellular S pyogenes after epithelial cell exposure to antibiotics commonly recommended for therapy was recently studied in a human laryngeal epithelial cell line (HEp2) using bacteriologic and electron microscopic evaluation.[4 ] S pyogenes survived intracellularly despite exposure of the streptococci-containing epithelial cells to penicillin. In contrast, ingested S pyogenes was killed after exposure of epithelial cells to either erythromycin or azithromycin. These observations strongly suggest that if the carrier state results from intraepithelial cell streptococci survival, the failure of penicillin to kill ingested S pyogenes may be related to a lack of effective penicillin entry into epithelial cells. These observations may have clinical implications for understanding carriers and managing S pyogenes infection. Frequency United States Upper respiratory tract infection is most common in the northern regions of the United States, especially during winter and early spring. By contrast, streptococcal skin infections occur most frequently during the summer (or year-round in warm climates), when the skin is exposed and abrasions and insect bites are more likely to occur. Interestingly, unique strains characterized by Erdem and colleagues appear to predominant in Hawaii,[5 ]and novel emm types are associated with invasive disease and streptococcal-related sequelae. Disease in neonates is uncommon, probably in part because of the effect of protective transplacentally acquired antibody. Prevalence of pharyngeal infection is highest in children older than 3 years. Indeed, group A streptococcal pharyngitis has been described as hazard in school-aged children.[6 ] S pyogenes also has the potential to produce outbreaks of disease in younger children in daycare. Evidence suggests that the frequency of severe, invasive group A streptococcal infections is increasing and that strains of streptococci with increased pathogenic potential are appearing. An increasing number of patients are being identified who have various unusually severe soft tissue infections associated with marked systemic toxicity, bacteremia, and shock. Factors responsible for the emergence of these more virulent strains of S pyogenes are not clearly defined, although many of these outbreaks appear to be clonal in nature. International Infections with group A Streptococcus are observed worldwide. Prevalence of streptococcal pyoderma is higher in regions near the tropics. Aside from this observation, no geographic barriers to infection with this ubiquitous organism are recognized. Rheumatic fever is most frequently observed in the age group most susceptible to group A streptococcal infections (ie, children aged 5-15 y). The attack rate following upper respiratory tract infection is approximately 3% in individuals with untreated or inadequately treated infection. Mortality/Morbidity Infection with group A streptococci leads to various clinical manifestations responsible for considerable morbidity and, with increasing frequency, mortality.[7 ]In addition, infection with this organism leads to postsuppurative sequelae, particularly acute rheumatic fever and poststreptococcal glomerulonephritis (PSGN). These disease manifestations are considered in Medical Care. Race Group A streptococcal infections are observed worldwide. Streptococcal pyoderma is a more common complication closer to tropical regions of the world. Otherwise, no racial or ethnic predispositions to infection with this organism are recognized. Sex In general, no sex-based differences are observed with this pathogen. Age Group A streptococcal infections may be observed in people of any age, although the prevalence of infection is higher in children, presumably because of the combination of multiple exposures (in school or daycare) and little immunity. Group A streptococcal pharyngitis is particularly common in school-aged children. Clinical History History, physical, and causes of group A streptococcal infections are reviewed by disease in Medical Care. Physical Infection with group A streptococci includes a wide variety of manifestations. Classic acute disease involves the skin and oropharynx, but any organ system may be involved. Complications can include toxic shock syndrome (TSS) and multiple-organ system disease. Long-term complications (nonsuppurative sequelae) include acute rheumatic fever (which, in turn, may include any of several major and minor manifestations involving the heart, joints, skin, and CNS) and poststreptococcal glomerulonephritis (PSGN). Causes Epidemiology Streptococcus pyogenes can be present on healthy skin for at least a week before lesions appear. Spread is via skin contact, not via the respiratory tract, although impetigo serotypes may colonize the throat. Person-to-person transmission is the route by which S pyogenes is primarily spread, although foodborne and waterborne outbreaks have been documented. Neither spread of organisms by fomites nor transmission from animals (eg, family pets) appears to play a significant role in contagion. S pyogenes is highly communicable and can cause disease in healthy people of all ages who do not have type-specific immunity against the specific serotype responsible for infection. Respiratory droplet spread is the major route for transmission of strains associated with upper respiratory tract infection, although skin-to-skin spread is known to occur with strains associated with streptococcal pyoderma. Children with untreated acute infections spread organisms by airborne salivary droplet and nasal discharge. The incubation period for pharyngitis is 2-5 days. Children are usually not infectious within 24 hours after appropriate antibiotic therapy has been started, an observation that has important implications for return to the daycare or school environment. Individuals who are streptococcal carriers (chronic asymptomatic pharyngeal and nasopharyngeal colonization) are not usually at risk of spreading disease to others because of the generally small reservoir of often-avirulent organisms. Fingernails and the perianal region can harbor streptococci and can play a role in disseminating impetigo. Multiple streptococcal infections in the same family are common. Both impetigo and pharyngitis are more likely to occur among children living in crowded homes and in suboptimal hygienic conditions. Differential Diagnoses Arthritis, Septic Bacteremia Bacterial Tracheitis Empyema Epiglottitis Fever in the Toddler Impetigo Kawasaki Disease Lymphadenitis Lymphadenopathy Mitral Valve Insufficiency Nephritis Osteomyelitis Pneumonia Rheumatic Fever Rheumatic Heart Disease Staphylococcus Aureus Infection Streptococcal Infection, Group A Toxic Shock Syndrome Other Problems to Be Considered Acute appendicitis Epstein-Barr virus Gastroenteritis Multiple-organ system disease Roseola Workup Laboratory Studies As noted, culture of group A streptococci is the criterion standard for diagnosis. Depending on disease manifestations, cultures of pharyngeal secretions, blood, cerebrospinal fluid, joint aspirate, leading edge aspirate of cellulitis, skin biopsy specimen, epiglottic secretions, bronchoalveolar lavage fluid, thoracocentesis fluid, or abscess fluid may be sources for locating the organism. In cases of suspected necrotizing fasciitis, a frozen section biopsy obtained in the operating room may be of great value in confirming the diagnosis and may aid in defining how much surgical debridement of devitalized tissue is necessary. As discussed elsewhere in this article, serologic assays (antistreptococcal antibodies) are a potential useful adjunct for diagnosis. Other ancillary laboratory tests (eg, CBC count, WBC count, erythrocyte sedimentation rate, C-reactive protein) may be useful depending on the manifestations of disease under consideration. This is discussed in a disease-by-disease fashion in Medical Care. Imaging Studies Various imaging studies may be warranted for streptococcal pneumonia, septic arthritis, osteomyelitis, brain abscess, and for complications of streptococcal infection, such as acute rheumatic fever or glomerulonephritis. Possible imaging studies include plain radiography, CT scanning, ultrasonography, echocardiography, and radioisotope renal scanning. For CNS manifestations, such as chorea or pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) syndrome, modalities such as MRI or positron emission tomography/single-photon emission CT (PET/SPECT) may be valuable. These are addressed on a disease-by-disease basis in Medical Care. Other Tests Other tests, depending on disease syndrome, can be very diverse in nature. For example, a histopathologic analysis of skin biopsy specimens, which may need to be analyzed intraoperatively, is warranted in cases of suspected necrotizing fasciitis. Calculation of creatinine clearance may be valuable in assessing the extent of renal dysfunction for nephritis. These issues are reviewed by disease in Medical Care. Procedures Necessary procedures for the management of the diverse nature of group A streptococcal infections may include endotracheal intubation, thoracocentesis, lumbar puncture, abscess or skin aspiration, and even surgical debridement of devitalized tissue. These issues are reviewed by disease in Medical Care. Histologic Findings As noted above, histologic analysis of skin biopsies may be an important tool in the diagnosis of streptococcal necrotizing fasciitis. In this setting, one of the hallmarks of the histologic findings is the absence of inflammatory cells, which suggests the necrotic, avascular nature of the affected tissue. Treatment Medical Care he approach to various acute streptococcal syndromes is described below, including diagnosis, clinical manifestations, and management. Streptococcal Pharyngitis Acute pharyngitis represents one of the most common reasons children are seen by a pediatrician. Yet, despite the common nature of the problem, few subjects engender more controversy than that of the diagnostic and therapeutic approach to the child with a sore throat. Many questions provoke disagreement on this topic, but some of the major points debated among clinicians include the following: Which children should be tested for streptococcal pharyngitis? How should children be tested for streptococcal pharyngitis? What treatment approach should be used for suspected streptococcal pharyngitis? In general, make decisions about laboratory testing and antibiotic therapy only after careful consideration of epidemiologic factors and clinical findings. The most important historic information in the evaluation of a sore throat is that of the presence or absence of other symptoms of upper respiratory tract infection. Children with streptococcal pharyngitis do not have cough, rhinorrhea, or symptoms of viral upper respiratory tract infection. Indeed, the diagnosis of streptococcal pharyngitis can effectively be ruled out on the basis of the clinical findings of marked coryza, hoarseness, cough, or conjunctivitis. However, although these are important exclusionary criteria, the pediatrician must be aware that signs and symptoms of streptococcal pharyngitis may otherwise be nonspecific and widely vary depending on patient age, severity of the infection, or timing of the illness. Relatively few localizing or constitutional symptoms may be present, such that the illness may be unrecognized (subclinical infection). Young infants do not present with classic pharyngitis. Streptococcal upper respiratory tract infections in infants and toddlers instead may be characterized by low-grade fever, anorexia, and a thick purulent nasal discharge (so-called "streptococcosis"). Conversely, some patients may be toxic, with high fever, malaise, headache, and severe pain upon swallowing. Streptococcal toxic shock can be associated with pharyngitis; however, this is rare. Vomiting and abdominal pain may be prominent early symptoms simulating gastroenteritis or even acute appendicitis. Hence, streptococcal pharyngitis should be considered in a child with acute onset of abdominal pain. Because streptococcal pharyngitis is chiefly a disease of winter and spring and primarily affects children older than 3 years, fewer throat cultures should be completed in the summer and in children younger than 3 years. Upon physical examination, children with classic group A streptococcal pharyngitis are more likely to demonstrate tonsillopharyngeal erythema, a red edematous uvula, palatal petechiae, and tender anterior cervical adenopathy than children with pharyngitis of other etiologies. Typically, tonsils are enlarged and erythematous with patchy exudate on the surface, although the presence of exudate is not pathognomonic for streptococcal pharyngitis and may be observed in the context of other bacterial and viral etiologies of pharyngitis, particularly Epstein-Barr virus. The papillae of the tongue may be red and swollen (so-called strawberry tongue). Cutaneous petechiae are not uncommon, and a scarlatiniform rash may be present (see Scarlet Fever). When the characteristic rash of scarlet fever is present, a clinical diagnosis can be made with increased confidence. However, consistently making the diagnosis of streptococcal pharyngitis on clinical grounds alone is difficult. Various clinical scoring systems has been devised to attempt to predict the results of subsequent throat cultures or antigen detection tests; however, at best, these scoring systems have no more than an 80% predictive value. Therefore, even the most experienced clinician should rely on bacteriologic confirmation of the diagnosis. Some clinicians express a reluctance to obtain diagnostic studies in children with sore throats, rationalizing this approach with the mistaken assumption that all febrile respiratory tract ailments require a course of antibiotic therapy. The ongoing crisis in antibiotic resistance and the urgent need to use a more judicious approach in antimicrobial prescribing practice should, hopefully, herald a return to appropriate diagnostic testing for group A streptococcal pharyngitis. The appropriate bacteriologic confirmation of the tentative diagnosis of streptococcal pharyngitis is disputed. Fifteen years after their introduction into clinical practice, controversy persists regarding the relative merits of antigen detection systems for Streptococcus pyogenes compared with traditional throat culture. Despite technologic improvements in rapid streptococcal testing, the throat culture remains the criterion standard for the diagnosis of streptococcal pharyngitis. If performed correctly, a throat swab cultured on a blood agar plate has a sensitivity rate of 9095% in detecting the presence of S pyogenes in the pharynx. This sensitivity depends on properly obtaining the specimen. When possible, a specimen should be obtained from the surface of both tonsils and from the posterior pharyngeal wall. Other areas of the oropharynx are not acceptable; in the uncooperative child, study of a culture that was obtained from areas of the mouth that are clearly known to be inadequate for culturing is difficult to justify. The culture should be examined at 24 hours postinoculation and again at 48 hours postinoculation. When considering the approach to bacteriologic diagnosis, emphasizing those patients who should not undergo throat culture is important. Cultures should not be obtained from children with nasal congestion, injected conjunctiva, and cough because these features indicate the presence of acute viral pharyngitis. A positive culture finding in this context only reflects chronic colonization (streptococcal carrier state). Although identifying and treating the streptococcal carrier may occasionally have merit, routinely obtaining cultures in children with symptoms suggestive of viral pharyngitis is not warranted and leads to unwarranted courses of antibiotic therapy. Although a negative throat culture finding essentially rules out the diagnosis of streptococcal pharyngitis, a positive culture finding unfortunately cannot be used to differentiate between acute infection and asymptomatic carriage. Some studies have reported that the degree of positivity of the culture may, by quantifying the load of organisms, assist in making this differentiation. However, in practice, assuming that all positive results in appropriately cultured patients represent streptococcal infection and accepting that some degree of overtreatment is inevitable is probably best. Sometimes families express concern regarding the delay of 24-48 hours that is required to obtain throat culture findings. Therefore, clinicians feel pressure to immediately initiate therapy, prior to obtaining the result of the culture. However, because treatment of group A streptococcal sore throat as long as 9 days after onset of symptoms still effectively prevents rheumatic fever, initiation of antibiotics is seldom of urgent importance. Early antibiotic therapy may have beneficial effects in relieving symptoms and allowing an earlier return to school or daycare; however, early antibiotic therapy may have disadvantages as well. Several controlled studies have shown that children receiving immediate antibiotic therapy are more likely to have symptomatic recurrences in the months following treatment than are children who delay the initiation of therapy by 48 hours. When the diagnosis of streptococcal pharyngitis seems particularly likely based on examination findings or when social factors necessitate an immediate decision about antibiotic therapy, the use of rapid antigen detection tests capable within minutes of identifying group A streptococci directly from the throat swab is a reasonable option in most practice settings. Most kits use antibodies for the detection of group A carbohydrate antigen. The indicator systems used are latex agglutination or enzyme immunoassay. Tests can be completed in a matter of minutes. Numerous studies have demonstrated that the currently available rapid streptococcal tests have a sensitivity of 70-90% compared with standard throat cultures. In contrast to their relatively low sensitivity, the specificity of these rapid tests has consistently been 90-100%. Therefore, if a rapid streptococcal test result is positive, a culture is not necessary, and appropriate antibiotic therapy can be immediately initiated. However, when a negative rapid test result is encountered, a standard throat culture should always be obtained. Streptococcal Skin Infections Superficial pyoderma is the most common form of skin infection caused by group A Streptococcus. Also referred to as streptococcal impetigo (or "impetigo contagiosa"), it occurs most commonly in tropical climates but can be highly prevalent in northern climates as well, particularly in the summer months. Risk factors that predispose to this infection include low socioeconomic status; low level of overall hygiene; and local injury to skin caused by insect bites, scabies, atopic dermatitis, and minor trauma. Colonization of unbroken skin precedes the development of pyoderma by approximately 10 days. This form of streptococcal infection is usually painless, and the patient is usually afebrile. Streptococcal impetigo usually has the highest prevalence in young children (aged 2-5 y). Infection spreads readily to other individuals from the skin lesions, and multiple occurrences within families are common. Streptococcal impetigo usually appears first as a discrete papulovesicular lesion surrounded by a localized area of redness. The vesicles rapidly become purulent and covered with a thick, confluent, honey-colored crust. The appearance of the lesions of streptococcal impetigo is in contrast to the classic bullous appearance of impetigo due to phage group II Staphylococcus aureus. However, recent evidence indicates that many cases of nonbullous impetigo are, in fact, mixed infections containing both S aureus and S pyogenes, and conclusions about etiology based on the clinical appearance of impetigo should be drawn with caution. Lesions are most commonly encountered on the face and extremities. If untreated, streptococcal impetigo is a mild but chronic illness, often spreading to other parts of the body. Regional lymphadenitis is common. The M types that give rise to streptococcal tonsillitis (ie, types 1, 3, 5, 6, 12, 18, 19, and 24) are rarely found in streptococcal impetigo. One of the streptococcal pyoderma-associated strains, the M49 strain, is very strongly associated with PSGN. Deeper soft tissue infections may occur following colonization of the skin with S pyogenes. A deeply ulcerated form of streptococcal impetigo, ecthyma, may complicate streptococcal impetigo. Ecthyma tends to be a more deep-seated and chronic form of streptococcal impetigo and is encountered mainly in the tropics. Streptococcal cellulitis is an acute rapidly spreading infection of skin and subcutaneous tissue, which can follow burns, wounds, surgical incisions, varicella infection, and mild trauma. Pain, tenderness, swelling and erythema, and systemic toxicity are common, and patients may have associated bacteremia. Careful serial examination is crucial because cellulitis may progress to necrotizing fasciitis (see Media file 1). Invasive soft tissue infection due to Streptococcus pyogenes. This child developed fever and soft tissue swelling on the fifth day of varicella-zoster infection. Leading edge aspirate of cellulitis grew S pyogenes. Although the patient responded to intravenous penicillin and clindamycin, operative debridement was necessary because of clinical suspicion of early necrotizing fasciitis. Perianal cellulitis and vaginitis should be considered in children who report perineal discomfort or vaginal discharge. Today, erysipelas is a relatively rare acute streptococcal infection involving the deeper layers of the skin and the underlying connective tissue. Skin over the affected area tends to be swollen, red, and exquisitely tender in contrast to streptococcal impetigo, which is usually painless. Superficial blebs may be present. The most characteristic finding in erysipelas, the sharply defined and slightly elevated border, helps to differentiate this entity from cellulitis, which has an indistinct border. At times, reddish streaks of lymphangeitis may project out from the margins of the lesion. Systemic toxicity is common. For both erysipelas and cellulitis, cultures obtained by leading edge needle aspirate of the inflamed area are warranted. [#targetScarlet]Scarlet Fever When a fine, diffuse, erythematous rash is present in the setting of acute streptococcal pharyngitis, the illness is called scarlet fever. The rash of scarlet fever is caused by the pyrogenic exotoxins (ie, SPE A, B, C, and F). The rash highly depends on toxin expression; preexisting humoral immunity to the specific SPE toxin prevents the clinical manifestations of scarlet fever. Recently, scarlet fever is apparently less common and is less virulent than in past decades; however, incidence is cyclic, depending on the prevalence of toxin-producing strains and the immune status of the population. Modes of transmission, age distribution of cases, and other epidemiologic features are similar to those for streptococcal pharyngitis. Scarlet fever rash usually appears within 24-48 hours after onset of symptoms, although it may appear with the first signs of illness. It is often initially noticed on the neck and upper chest as a diffuse, finely papular, erythematous eruption producing a bright red discoloration of the skin, which blanches on pressure. The texture is that of fine sandpaper. The flexor skin creases, particularly in the antecubital fossae, may be unusually prominent (ie, Pastia lines). The area around the mouth is pale, creating the appearance of circumoral pallor. In severe cases, small vesicular lesions (ie, miliary sudamina) may appear on the abdomen, hands, and feet. Toward the end of the first week of illness, the rash begins to fade and is followed by a desquamation over the trunk, which progresses to the hands and feet. Typical scarlet fever is not generally difficult to diagnose, but it may be confused with roseola, Kawasaki syndrome, drug eruptions, and toxigenic S aureus infections. A history of recent exposure to another individual (eg, classroom or household contact) with streptococcal infection is a helpful clue. Isolation of S pyogenes from the pharynx confirms the diagnosis in uncertain cases, and serologic evidence of recent group A streptococcal infection may be present (ASO or anti-DNAse B antibody response). Other Miscellaneous Streptococcal Infections Suppurative complications from the spread of streptococci to adjacent structures were very common in the preantibiotic era. Cervical adenitis, peritonsillar abscess, retropharyngeal abscess, otitis media, mastoiditis, and sinusitis still occur in children in whom the primary illness has gone unnoticed or in whom treatment of the pharyngitis has been inadequate because of noncompliance. S pyogenes is an occasional etiology of pneumonia and is an important etiology of parapneumonic effusion. Acute hematogenous osteomyelitis is an important complication of streptococcal infection. Isolated bacteremia, meningitis, and endocarditis are described but appear to be rare manifestations of acute infection. Invasive Streptococcal Infections Invasive infections with S pyogenes have been encountered with increased frequency in recent years. These may manifest as either necrotizing fasciitis or streptococcal toxic shock syndrome (TSS). Necrotizing fasciitis Necrotizing fasciitis caused by S pyogenes (so-called streptococcal gangrene) is an acute, rapidly progressive, severe, deep-seated infection of the subcutaneous tissue associated with extensive destruction of superficial and deep fascia. Diffuse erythematous swelling heralds the onset, with exquisite pain at the affected site. Indeed, severe excruciating pain that seems inconsistent with the observed clinical findings should strongly suggest the possibility of this diagnosis. As the lesion progresses (approximately 48-72 h), the skin becomes bluish and dusky, and bullae containing yellow or hemorrhagic fluid appear. By the fourth to fifth day, frank gangrene is present, and extensive sloughing of skin occurs. Surgical debridement of necrotic tissue is a crucial adjunct to management. Differentiation between streptococcal cellulitis and necrotizing fasciitis can be difficult, and careful serial physical examination is crucial. Consultation with a surgeon early in the course of infection is essential because debridement is often lifesaving. If diagnosis is not certain on clinical grounds, a biopsy with frozen section may be useful. Histopathology commonly reveals both microbial and neutrophilic infiltration of deep dermal and superficial fascial layers of skin, with resultant thrombosis, vasculitis, and necrosis. Although any part of the body may be affected, streptococcal fasciitis usually begins on an extremity. It may begin at a site of trivial or inapparent trauma, or it may follow cuts, burns, penetrating injuries, or blunt trauma. A major risk factor for development of streptococcal necrotizing fasciitis is a history of recent varicella-zoster virus (VZV) infection (see Media file 1). The risk of varicella-associated necrotizing fasciitis should decrease with the implementation of routine childhood immunization against VZV. Streptococcal toxic shock syndrome Streptococcal TSS is characterized by hypotension and multiple-organ failure. Considerable overlap occurs with streptococcal necrotizing fasciitis, insofar as most cases occur in association with soft tissue infections; however, streptococcal TSS may occur in association with other focal streptococcal infections, including pharyngeal infection. As noted above, the pathogenesis of streptococcal TSS appears to be related in part to the ability of certain (ie, A, C, F) streptococcal pyogenic exotoxins (SPEs) to function as superantigens. Multiple-organ system disease is common and manifests as renal impairment, occurring in approximately 80% of patients, and hepatic dysfunction, occurring in 65% of patients. Criteria proposed by the Working Group on Severe Streptococcal Infections for the diagnosis of streptococcal toxic shock are outlined as follows:[8 ] Isolation of group A Streptococcus o From a sterile site o From a nonsterile body site Clinical signs of severity (Two or more of the following clinical and laboratory abnormalities are required.) o Renal impairment o Coagulopathy o Liver abnormalities o Acute respiratory distress o Extensive tissue necrosis (necrotizing fasciitis) o Erythematous rash Definite case - Isolation of group A Streptococcus from a sterile site plus compatible clinical signs Probable case - Isolation of group A Streptococcus from a nonsterile body site plus compatible clinical signs Surgical Care Necessary procedures for management of the diverse nature of group A streptococcal infections may include endotracheal intubation, thoracocentesis, lumbar puncture, abscess or skin aspirate, prompt surgical drainage, and even surgical debridement of devitalized tissue, fasciotomy, or amputation (see Medical Care). Some children with recurrent streptococcal pharyngitis (7 culture-proven episodes in the preceding year) may benefit from tonsillectomy. Consultations Surgeon (for necrotizing fasciitis and bone and joint infections) Critical care specialist (for epiglottitis and TSS) Nephrologist (for poststreptococcal glomerulonephritis [PSGN]) Neurologist (for chorea and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections [PANDAS] syndrome) Infectious diseases specialist (for assistance in differential diagnosis and broad management issues) Cardiologist (for carditis) Dermatologist (for skin conditions) Pathologist (for analysis of biopsies and other intraoperative specimens) Medication Treatment approaches for group A streptococcal infections vary depending on the clinical syndrome. Penicillin therapy, in general, remains the treatment of choice in most situations. Remarkably, no penicillin-resistant strains of S pyogenes have yet been encountered in clinical practice.[9 ]Therefore, penicillin remains the drug of choice for pharyngeal infections as well as for complicated or invasive infections, except in individuals who are allergic to penicillin. In those patients who are allergic to penicillin, once-daily therapy with amoxicillin and azithromycin (50 mg/kg) is recommended.[10 ]Approaches to antibiotic therapy of various streptococcal syndromes are considered below. Antibiotics Empiric antimicrobial therapy must be comprehensive and should cover all likely pathogens in the context of the clinical setting. Penicillin VK ( Pfizerpen, Pen-Vee K, Beepen-VK) Inhibits the biosynthesis of cell wall mucopeptide. Bactericidal against sensitive organisms when adequate concentrations are reached, and most effective during the stage of active multiplication. Inadequate concentrations may produce only bacteriostatic effects. For streptococcal pharyngitis, the PO antibiotic of choice is penicillin VK (phenoxymethyl penicillin). Penicillin VK is preferable to penicillin G because of its acid stability, allowing it to be dosed without regard to meals. The most common reason for penicillin failure is noncompliance. The drug is often discontinued before the 10-d course is completed because children usually appear to have recovered in 3-4 d. When PO treatment is prescribed, the necessity of completing a full course of therapy must be emphasized. Even in compliant patients, recent reports suggest penicillin fails to eradicate S pyogenes from about 15% of treated patients. Many theories have been proposed to explain these apparent penicillin failures. The presence of beta-lactamase–producing normal flora (particularly organisms such as mouth anaerobes) is proposed as a potential mechanism by which penicillin may become inactivated. However, the clinical significance of this theory has never been conclusively demonstrated. Many of the failures of penicillin therapy are more likely to occur in studies where streptococcal pharyngitis has not been defined rigorously enough, and some of these patients may, in fact, be streptococcal carriers who had viral pharyngitis at study onset. Dosing Adult 250-500 mg PO bid Pediatric 40 mg/kg/d PO or 250 mg PO bid/tid Interactions Probenecid may increase effectiveness by decreasing clearance; tetracyclines are bacteriostatic, causing a decrease in the effectiveness of penicillins when administered concurrently Contraindications Documented hypersensitivity Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Caution in renal impairment Penicillin G benzathine (Bicillin LA) Interferes with synthesis of cell wall mucopeptides during active multiplication, which results in bactericidal activity. If noncompliance with PO therapy seems likely, parenteral therapy is indicated. Formulation is painful when administered IM, and it is often combined with penicillin G procaine to minimize discomfort at the injection site. When this combination is used in a single injection, take care to ensure that an adequate amount of penicillin G benzathine is administered. The combination of 900,000 U of penicillin G benzathine and 300,000 U of penicillin G procaine is satisfactory for most children. Dosing Adult 1.2 million U IM for 1 dose Pediatric <27 kg: 600,000 U IM for 1 dose >27 kg: Administer as in adults Interactions Probenecid can increase penicillin effectiveness by decreasing clearance; coadministration with tetracyclines can decrease effectiveness of penicillin Contraindications Documented hypersensitivity Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Caution in impaired renal function Erythromycin (EES, Ery-Tab, E-Mycin) Inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNA-dependent protein synthesis to arrest. For treatment of staphylococcal and streptococcal infections. In children, age, weight, and severity of infection determine proper dosage. When bid dosing is desired, half-total daily dose may be taken q12h. For more severe infections, double the dose. PO erythromycin is an acceptable alternative for patients allergic to penicillin or cephalosporin antibiotics and is effective in the treatment of streptococcal pharyngitis. Erythromycin estolate and erythromycin ethylsuccinate are both effective, although note local antibiotic resistant rates because up to 5% of isolates of S pyogenes may be erythromycin resistant. Dosing Adult 250 mg erythromycin stearate/base (or 400 mg ethylsuccinate) q6h PO 1 h ac or 500 mg q12h; alternatively, 333 mg q8h and increase to 4 g/d depending on severity of infection Pediatric 30-50 mg/kg/d (15-25 mg/lb/d) PO divided q6-8h Interactions Coadministration may increase toxicity of theophylline, digoxin, carbamazepine, and cyclosporine; may potentiate anticoagulant effects of warfarin; coadministration with lovastatin and simvastatin increases risk of rhabdomyolysis Contraindications Documented hypersensitivity; hepatic impairment Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Caution in liver disease; estolate formulation may cause cholestatic jaundice; GI adverse effects are common (administer doses pc); discontinue use if nausea, vomiting, malaise, abdominal colic, or fever occur Clarithromycin (Biaxin) Inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNA-dependent protein synthesis to arrest. Similar susceptibility profile to erythromycin but has fewer adverse effects. Dosing Adult 250-500 mg PO q12h Pediatric 15 mg/kg/d PO divided bid Interactions Toxicity increases with coadministration of fluconazole and pimozide; clarithromycin effects decrease and GI adverse effects may increase with coadministration of rifabutin or rifampin; may increase toxicity of anticoagulants, cyclosporine, tacrolimus, digoxin, omeprazole, carbamazepine, ergot alkaloids, triazolam, and HMG CoA-reductase inhibitors; plasma levels of certain benzodiazepines may increase, prolonging CNS depression; arrhythmias and increase in QTc intervals occur with disopyramide; coadministration with omeprazole may increase plasma levels of both agents Contraindications Documented hypersensitivity; coadministration of pimozide Precautions Pregnancy C - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus Precautions Coadministration with ranitidine or bismuth citrate is not recommended with CrCl <25 mL/min; administer half dose or increase dosing interval if CrCl <30 mL/min; diarrhea may be sign of pseudomembranous colitis; superinfections may occur with prolonged or repeated antibiotic therapies Azithromycin (Zithromax) Similar susceptibility profile to erythromycin, but has fewer adverse effects. Treats mild-tomoderate microbial infections. Dosing Adult Day 1: 500 mg PO Days 2-5: 250 mg PO qd Pediatric 12 mg/kg/d PO for 5 d Interactions May increase toxicity of theophylline, warfarin, and digoxin; effects are reduced with coadministration of aluminum antacids, magnesium antacids, or both; nephrotoxicity and neurotoxicity may occur when coadministered with cyclosporine Contraindications Documented hypersensitivity; hepatic impairment; coadministration with pimozide Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Site reactions can occur with IV route; bacterial or fungal overgrowth may result with prolonged antibiotic use; may increase hepatic enzymes and cholestatic jaundice; caution in patients with impaired hepatic function, prolonged QT intervals, or pneumonia; caution in hospitalized, geriatric, or debilitated patients Cephalexin (Keflex, Biocef) First-generation cephalosporin arrests bacterial growth by inhibiting bacterial cell wall synthesis. Bactericidal activity against rapidly growing organisms. Primary activity against skin flora; used for skin infections or prophylaxis in minor procedures. PO cephalosporins are effective in the treatment of streptococcal pharyngitis. Short-course regimens of PO cephalosporin therapy have been studied and offer obvious advantages from a compliance perspective. However, this must be balanced against the higher cost and unnecessarily broad spectrum of these agents. Dosing Adult 250 mg PO q6h or 500 mg PO q12h Pediatric 25-50 mg/kg/d PO divided q6h Interactions Coadministration with aminoglycosides increase nephrotoxic potential Contraindications Documented hypersensitivity Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Adjust dose in renal impairment Clindamycin (Cleocin) Lincosamide for treatment of serious skin and soft tissue staphylococcal infections. Also effective against aerobic and anaerobic streptococci (except enterococci). Inhibits bacterial growth, possibly by blocking dissociation of peptidyl tRNA from ribosomes, causing RNAdependent protein synthesis to arrest. Patients with invasive group A streptococcal infections (eg, necrotizing fasciitis, TSS, sepsis) should be treated with IV penicillin in combination with clindamycin. Because the pathophysiology of invasive group A streptococcal infection is largely toxin mediated, the use of protein synthesis inhibitor (eg, clindamycin) offers a theoretical advantage. Furthermore, in vivo evidence of the lack of efficacy of penicillin in deep tissue infections has been observed in animal models. This effect, first described by Eagle in 1952, appears to occur because of high inoculum of organisms encountered in overwhelming infections (eg, necrotizing fasciitis, myositis, sepsis). Large concentrations of organisms lead to rapid attainment of the stationary growth phase, which is associated with decreased expression of cell wall penicillin-binding proteins (PBPs), the molecular targets of penicillin. Decreased expression of PBPs in deep tissue infections with group A streptococci appears to render penicillin less effective. In contrast, clindamycin retains efficacy. Vigorous supportive care, including fluids, pressors, and mechanical ventilation, is also a critical aspect of management of invasive streptococcal skin and soft tissue infections. Prompt surgical drainage, debridement, fasciotomy, or amputation may be indicated. Differentiating a streptococcal carrier with recurrent viral infection from a child with recurrent streptococcal pharyngitis may be difficult. Although most streptococcal carriers do not require medical intervention, situations arise in which eradication of the carrier state is desirable (eg, families in with an inordinate amount of anxiety about streptococci, families in which ping-pong spread has been occurring, when tonsillectomy is considered only because of chronic carriage). A course of clindamycin has been shown to be highly effective in eradicating the carrier state and should be tried in patients with recurrent or frequent episodes of culture-proven pharyngitis. Some children with recurrent streptococcal pharyngitis (7 culture-proven episodes in the preceding y) may benefit from tonsillectomy. Dosing Adult 150-450 mg/dose PO q6-8h; not to exceed 1.8 g/d Pediatric 20 mg/kg/d PO divided tid for 10 d Interactions Increases duration of neuromuscular blockade induced by tubocurarine and pancuronium; erythromycin may antagonize effects of clindamycin; antidiarrheals may delay absorption of clindamycin Contraindications Documented hypersensitivity; regional enteritis; ulcerative colitis; hepatic impairment; antibiotic-associated colitis Precautions Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals Precautions Adjust dose in severe hepatic dysfunction; no adjustment necessary in renal insufficiency; associated with severe and possibly fatal colitis Follow-up Further Inpatient Care Further inpatient care may be necessary in patients with group A streptococcal infections for rehabilitative reasons (eg, chorea, neuropsychiatric manifestations of infection) or for debilitating arthritis. Consultation with a physical medicine and rehabilitation (PMR) physician, neurologist, or rheumatologist may be useful in these situations. Further Outpatient Care Outpatient follow-up care with infectious diseases specialists (management of long-term therapy or prophylaxis for acute rheumatic fever), surgeons, neurologists, rheumatologists, and nephrologists may be important. The primary care physician must be closely involved in managing and coordinating longterm special services. Deterrence/Prevention Prophylaxis against streptococcal infection o Long-term antibiotic therapy to prevent streptococcal infection is indicated for patients with a history of acute rheumatic fever or rheumatic heart disease. The recommended regimen is an injection every 3-4 weeks with 1.2 million IU of benzathine penicillin G, 250 mg of oral penicillin V twice a day, or 0.5-1 g of sulfadiazine daily. o The role of prophylaxis for household contacts of individuals with either acute streptococcal disease or nonsuppurative complications is uncertain. Some authorities recommend that cultures be obtained from all contacts if a family history of rheumatic fever is noted or when a patient with acute glomerulonephritis is identified. o An alternative approach is to treat all household contacts in the setting of acute poststreptococcal glomerulonephritis (PSGN) in an effort to eradicate household transmission of nephritogenic strains. For invasive group A streptococcal infections (eg, necrotizing fasciitis, toxic shock syndrome [TSS]), no data are available on which to base assessment of risk to household contacts. However, because of the devastating nature of these infections and the observation that invasive disease may be due to clonal outbreaks of more virulent strains, empiric antibiotic therapy of household contacts seems warranted. Prospects for streptococcal vaccines o Apart from rheumatic fever prophylaxis and the prevention of intrafamily spread, few strategies are available to prevent streptococcal infection. o A streptococcal vaccine could offer promise for prevention of disease, but an effective vaccine would have to provide protection from multiple serotypes. Furthermore, theoretical concern that vaccine-induced antibodies could injure host tissue and precipitate rheumatic fever is recognized. o Multivalent vaccines that contain multiple M protein peptide epitopes have been engineered and show efficacy in animal models but have not yet entered clinical trials.[11 ] Complications Acute rheumatic fever and acute PSGN are the classic nonsuppurative complications of S pyogenes infections. Although the link between group A streptococcal infections and these complications has been clearly established, the mechanism or mechanisms through which the injury is produced are incompletely defined. Acute rheumatic fever o During the 1960s and 1970s, this disease nearly disappeared in the United States, although it continued unabated in developing countries. This decline in disease was largely attributed to careful disease surveillance and initiation of prompt aggressive antibiotic therapy in primary care practice. However, in 1985, several multifocal outbreaks of rheumatic fever occurred in several parts of the United States. In contrast with earlier outbreaks in this country, most of the patients were o o o o o white, middle-class children from rural and suburban communities who had good access to health care. This unexplained resurgence in acute rheumatic fever underscores the point that a great deal remains to be learned about the pathogenesis of this disease. Epidemiologically, considerable evidence supports the link between group A streptococcal infections of the upper respiratory tract and acute rheumatic fever, although only certain M group serotypes (ie, 1, 3, 5, 6, 18, 24) are associated with this complication. Very mucoid strains, particularly strains of M type 18, have appeared in numerous communities prior to the appearance of rheumatic fever. Rheumatic fever is most frequently observed in children aged 5-15 years (the age group most susceptible to group A streptococcal infections). The attack rate following upper respiratory tract infection is approximately 3% for individuals with untreated or inadequately treated infection. The latent period between the group A streptococcal infection and the onset of rheumatic fever varies from 2-4 weeks. In contrast to PSGN, which may follow either pharyngitis or streptococcal pyoderma, rheumatic fever can occur only after an infection of the upper respiratory tract. Despite the depth of knowledge about the molecular microbiology of Streptococcus pyogenes that has accumulated in recent years, the pathogenesis of acute rheumatic fever remains unknown. A direct effect of a streptococcal extracellular toxin, in particular streptolysin O, may be responsible for the pathogenesis of acute rheumatic fever, according to some hypotheses. Observations that streptolysin O is cardiotoxic in animal models support this hypothesis, but linking this toxicity to the valvular damage observed in acute rheumatic fever has been difficult. A more popular hypothesis is that an abnormal host immune response to some component of the group A Streptococcus is responsible. The group A streptococcal M protein shares certain amino acid sequences with some human tissues, and this has been proposed as a source of cross-reactivity between the organism and human host that could lead to an immunopathologic immune response. Also, antigenic similarity between the group-specific polysaccharide of S pyogenes and glycoproteins found in human and bovine cardiac valves has been recognized, and patients with acute rheumatic fever have prolonged persistence of these antibodies compared with controls with uncomplicated pharyngitis. Other group A streptococcal antigens appear to cross-react with cardiac sarcolemma membranes. As a result of this molecular mimicry, during the course of the host's immune response to the group A streptococci, the host's antigens may be mistaken as foreign; this leads to an inflammatory cascade with resultant tissue damage. In patients with acute rheumatic fever with Sydenham chorea, common antibodies to antigens found in the S pyogenes cell membrane and the caudate nucleus of the brain are present, further supporting the concept of an aberrant autoimmune response in the development of acute rheumatic fever. Recently, interest in whether such autoimmune responses may play a role in the pathogenesis of the pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) syndrome has been considerable, although further work is necessary to establish the link between streptococcal infections and these syndromes. Differences in genetic susceptibility apparently play an important role in the likelihood of developing poststreptococcal sequelae, although the exact nature of the genetic predisposition remains undefined. o Acute rheumatic fever is largely a clinical diagnosis best established by careful physical examination. The Jones criteria for the diagnosis are outlined in Rheumatic Fever. Few patients with acute rheumatic fever have positive throat culture or rapid streptococcal antigen test findings at the time of presentation. o Because the isolation or identification of group A streptococci from a throat swab does not distinguish between a person with acute streptococcal infection and a person who is a streptococcal carrier, the best evidence of an antecedent streptococcal infection is a serologic response to the organism. An elevated streptococcal antibody titer can be used as serologic evidence of a recent group A streptococcal infection. Serial samples should be obtained because identification of a rising titer is particularly helpful. The most commonly used streptococcal antibody test is the ASO titer, although anti-DNase B and antihyaluronidase assays, which can be measured as a part of a panel of streptococcal antibodies referred to as the streptozyme panel, are also helpful. When 2 or more different streptococcal antibody tests are performed, an increased titer is found within the first few months of onset in most instances of acute rheumatic fever. Acute glomerulonephritis o Glomerulonephritis can follow group A streptococcal infections of either the pharynx or the skin, and incidence varies with the prevalence of so-called nephritogenic strains of group A streptococci in the community. Type 12 is the most frequent M serotype that causes PSGN after pharyngitis, and M type 49 is the type most commonly related to pyoderma-associated nephritis. The latent period between group A streptococcal infection and the onset of glomerulonephritis varies from 1-2 weeks. o Pathogenesis appears to be immunologically mediated. Immunoglobulins, complement components, and antigens that react with streptococcal antisera are present in the glomerulus early in the course of the disease, and antibodies elicited by nephritogenic streptococci are postulated to react with renal tissue in such a way as to promote glomerular injury. In contrast to acute rheumatic fever, recurrences of PSGN are rare. Diagnosis of PSGN is based on clinical history, physical examination findings, and confirmatory evidence of recent streptococcal infection (see Glomerulonephritis, Poststreptococcal). o Even in the absence of bacteriologic confirmation of S pyogenes, the presence of skin lesions compatible with streptococcal impetigo is highly suggestive, and elevated streptococcal antibody titers in the setting of a hypocomplementemic nephritis is essentially diagnostic of PSGN. Over the past several decades, incidence of acute PSGN in the United States has been steadily declining. Patient Education Educate patients with acute rheumatic fever on the need for long-term penicillin prophylaxis. For excellent patient education resources, visit eMedicine's Ear, Nose, and Throat Center and Women's Health Center. Also, see eMedicine's patient education articles Sore Throat, Strep Throat and Toxic Shock Syndrome. Miscellaneous Medicolegal Pitfalls Physicians must be aware and concerned about the potential for life-threatening complications presented by group A streptococcal infections. Even seemingly minor infections (eg, pharyngitis, impetigo) may lead to fatal toxic shock syndrome (TSS). Unusually ill-appearing children require aggressive inpatient evaluation and treatment. Streptococcal infections superimposed on varicella zoster virus (VZV) infection (chicken pox) represent a particularly high-risk situation. Aggressive treatment of such infections and close follow-up care is essential.