History of Parenteral Nutrition
advertisement
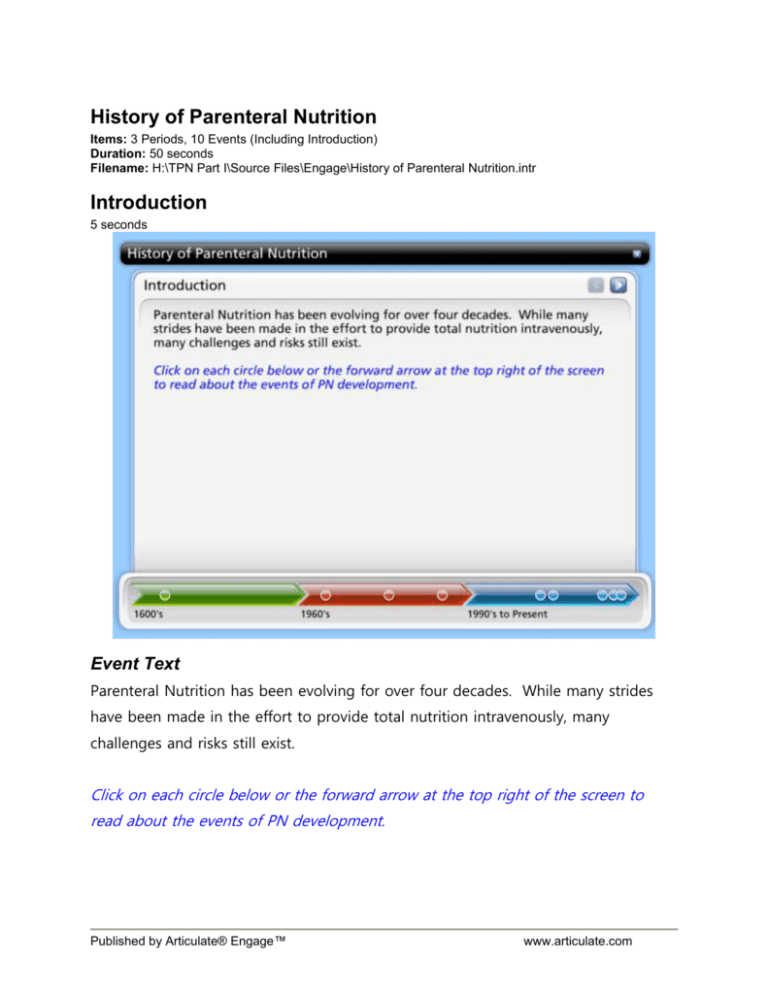
History of Parenteral Nutrition Items: 3 Periods, 10 Events (Including Introduction) Duration: 50 seconds Filename: H:\TPN Part I\Source Files\Engage\History of Parenteral Nutrition.intr Introduction 5 seconds Event Text Parenteral Nutrition has been evolving for over four decades. While many strides have been made in the effort to provide total nutrition intravenously, many challenges and risks still exist. Click on each circle below or the forward arrow at the top right of the screen to read about the events of PN development. Published by Articulate® Engage™ www.articulate.com 1600's 1616 5 seconds Event Text In 1616, circulation of blood in the human body is discovered. Published by Articulate® Engage™ www.articulate.com Reference: Dudrick SJ. J Am Coll Nutr 2009;28(3):243-251 Published by Articulate® Engage™ www.articulate.com 1960's Early 1960's 5 seconds Event Text Prior to the availability of IV diuretics (chlorothiazide) in 1962, the concept of intravenous nutrition had been abandoned due to the high volumes required for isotonicity of infused solutions. Prior to chlorothiazide, the existing diuretics were nephrotoxic at doses required when used for long periods of time. Published by Articulate® Engage™ www.articulate.com Reference: Dudrick SJ. J Am Coll Nutr 2009;28(3):243-251 Published by Articulate® Engage™ www.articulate.com Mid-1960's 5 seconds Event Text In the mid-1960's, intravenous nutrition advanced significantly due to extensive laboratory research on parenteral formulations in Beagle puppies. Through this work, sole nutritional support for growth, development and metabolic support was demonstrated. Published by Articulate® Engage™ www.articulate.com Reference: Dudrick SJ. J Am Coll Nutr 2009;28(3):243-251 Published by Articulate® Engage™ www.articulate.com Late-1960's 5 seconds Event Text Laboratory studies in Beagle puppies provide the foundation for successful trials in both adult and pediatric human cases. • Six adults at The Hospital of the University of Pennsylvania were the first to receive the basic nutrient solution developed as a modified version of the puppy formulation - all six were surgical patients discharged in good overall condition despite originally grave prognoses related to malnutrition. • One newborn, following an extensive resection of more than 95% of her bowel due to near-total small bowel atresia , survives for 22 months and achieves normal activity and development for her age while nourished via parenteral nutrition. Although she eventually died, the experience scientists and clinicians gained during her management is still unparalleled. Published by Articulate® Engage™ www.articulate.com Reference: Dudrick SJ. J Am Coll Nutr 2009;28(3):243-251 Published by Articulate® Engage™ www.articulate.com 1990's to Present 1994 5 seconds Event Text FDA Safety Alert of April 1994 Two patients die after receiving peripheral total nutrition admixture (TNA). Autopsies revealed amorphous deposits of calcium-phosphate in the pulmonary vasculature. Published by Articulate® Engage™ www.articulate.com Reference: Food and Drug Administration.Safety Alert: Hazards of precipitation associated with parenteral nutrition. Am J Hosp Pharm 1994;51:1427-1428 Published by Articulate® Engage™ www.articulate.com 1995 5 seconds Event Text American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) establishes the National Advisory Group (NAG) on Standards and Practice Guidelines for Parenteral Nutrition. The NAG was formed to identify problem areas in parenteral nutrition (PN) therapy specifically related to pharmacy practice after multiple deaths and injuries were reported. Published by Articulate® Engage™ www.articulate.com Reference: National Advisory Group on Standards and Practice Guidelines for Parenteral Nutrition. JPEN J Parenter Enteral Nutr 1998;22:49-66 Published by Articulate® Engage™ www.articulate.com 1997 5 seconds Event Text In January 1997, the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors approves "Safe Practices for Parenteral and Nutrition Formulations" to become the standard of practice for the provision of parenteral nutrition in ALL health care settings. The guidelines provide information on 1) labeling, 2) standard nutrient ranges, 3) extemporaneous compounding and 4) stability & compatability of PN formulations. Published by Articulate® Engage™ www.articulate.com Reference: National Advisory Group on Standards and Practice Guidelines for Parenteral Nutrition. JPEN J Parenter Enteral Nutr 1998;22:49-66 Published by Articulate® Engage™ www.articulate.com 2004 5 seconds Event Text The 1997 publication focused on problem areas in PN therapy specifically related to pharmacy practice. In 2004, a revised "Safe Practices for Parenteral Nutrition" was published by A.S.P.E.N. to encompass the interdisciplinary nature of PN and expanded to include the following topics: 1. Ordering of Parenteral Nutrition 2. Additional information on nutrient ranges to provide dosage recommendations beyond normal requirements 3. Additional information on filtration and administration of PN "Safe Practices for Parenteral Nutrition" can be reviewed by clicking on the "Attachments" tab at the top right of the screen. Published by Articulate® Engage™ www.articulate.com Reference: Task Force for the Revision of Safe Practices for Parenteral Nutrition. JPEN J Parenter Enteral Nutr 2004;28:S29-S70 Published by Articulate® Engage™ www.articulate.com 2011 5 seconds Event Text A total of 19 patients from six hospitals were adversely affected after receiving PN contaminated with Serratia marcescens bacteria. Nine of the 19 affected patients had died, as of April 7, 2011, although the CDC had not confirmed that the deaths were a direct cause of the contaminated PN. The ISMP Medication Safety Alert "TPN-Related Deaths Call for FDA Guidance and Pharmacy Board Oversight of USP Chapter <797>" can be reviewed by clicking on the "Attachments" tab at the top right of the screen. Reference: Published by Articulate® Engage™ www.articulate.com Accessed http://www.ismp.org/Newsletters/acutecare/articles/20110407.asp on July 8, 2011 Published by Articulate® Engage™ www.articulate.com