Supplementary Material for:
advertisement

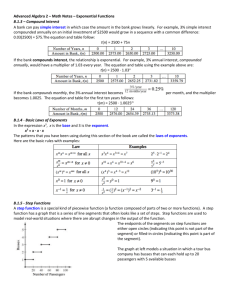
Supplementary Material for: Synthesis, Structure, Physicochemical Characterization and Electronic Structure of Thio-Lithium Super Ionic Conductors, Li4GeS4 and Li4SnS4 Joseph H. MacNeil,1 Danielle M. Massi,2 Jian-Han Zhang2, Kimberly A. Rosmus,2 Carl D. Brunetta,2 Taylor A. Gentile,2 and Jennifer A. Aitken*2 1 2 Department of Chemistry, Chatham University, 1 Woodland Road, Pittsburgh, PA 15232, USA Department of Chemistry and Biochemistry, Duquesne University, 600 Forbes Ave., Pittsburgh, PA 15282, USA *author to whom correspondence should be addressed. Email: aitkenj@duq.edu Phone:(412) 396-1670 Fax: (412) 396-5683 1 Table of Contents Source and purity of reagent chemicals 3 Selected interatomic distances (Å) and angles (°) for Li4GeS4. 4 Selected interatomic distances (Å) and angles (°) for Li4SnS4. 5 Symmetry equivalent positions for room temperature and 100K structures of Li4SnS4. 6-8 Comparison of fractional atomic coordinates for structure reports of Li4GeS4. 9-10 X-Ray Powder Diffractogram for Li4GeS4. 11 X-Ray Powder Diffractogram for Li4SnS4. 12 2 Source and Purity of Reagent Chemicals The chemicals in this work were used as obtained except for the germanium metal, which was first pulverized using an impact mortar and pestle and then ground in a ceramic mortar and pestle before use. Li2S powder 98% (99.9%-Li) Strem Chemicals Inc., Newburyport, MA Zn power 99.999% Strem Chemicals Inc., Newburyport, MA Sn powder 99.999% Strem Chemicals Inc., Newburyport, MA Fe powder 99.99% Strem Chemicals Inc., Newburyport, MA Ge metal pieces 99.999% Strem Chemicals Inc., Newburyport, MA sublimed S powder 99.5% Fisher Scientific Pittsburgh, PA methanol ACS grade BDH, Bridgeport, NJ ethanol 200 proof, ACS grade PHARMCO-APPER, Brookfield, CN heptane 99% Acros Organics, Morris Plains, NJ 3 Selected interatomic distances (Å) and angles (°) for Li4GeS4. Li(1)-S(3) 2.5570(5) × 2 S(3)-Li(1)-S(2) 87.27(2) × 2 Li(1)-S(2) 2.5893(6) × 2 S(3)-Li(1)-S(2) 92.73(2) × 2 Li(1)-S(1) 2.9092(6) × 2 S(1)-Li(1)-S(2) 86.91(2) × 2 Li(1)-Li(3) 2.756(2) × 2 S(1)-Li(1)-S(3) 88.70(2) × 2 Li(1)-Ge(1) 3.1718(6) × 2 S(1)-Li(1)-S(3) 91.30(2) × 2 Li(1)-Li(2) 3.251(2) × 2 S(1)-Li(1)-S(2) 93.09(2) × 2 S(1)-Li(1)-S(1) 180.0 S(2)-Li(1)-S(2) 180.00(1) S(3)-Li(1)-S(3) 180.0 Li(2)-S(2) 2.450(2) × 2 S(1)-Li(2)-S(3) 92.61(8) Li(2)-S(1) 2.493(3) S(1)-Li(2)-S(2) 107.93(7) × 2 Li(2)-S(3) 2.498(3) S(2)-Li(2)-S(2) 114.2(1) Li(2)-Ge(1) 3.022(3) S(2)-Li(2)-S(3) 115.65(7) × 2 Li(3)-S(2) 2.384(2) S(2)-Li(3)-S(3) 93.19(7) Li(3)-S(1) 2.405(2) S(1)-Li(3)-S(2) 98.97(8) Li(3)-S(3) 2.550(2) S(1)-Li(3)-S(3) 104.39(8) Li(3)-S(2) 2.576(3) S(2)-Li(3)-S(2) 109.75(9) S(1)-Li(3)-S(2) 119.98(9) S(2)-Li(3)-S(3) 124.42(9) Ge(1)-S(2) 2.2087(5) × 2 S(2)-Ge(1)-S(3) 106.36(1) × 2 Ge(1)-S(1) 2.2174(8) S(1)-Ge(1)-S(3) 108.57(1) Ge(1)-S(3) 2.2279(7) S(2)-Ge(1)-S(2) 111.47(2) S(2)-Ge(1)-S(1) 111.87(1) × 2 4 Selected interatomic distances (Å) and angles (°) for Li4SnS4. Li(1)-S(3) 2.6518(6) × 2 S(3)-Li(1)-S(2) 88.59(2) × 2 Li(1)-S(2) 2.6771(7) × 2 S(3)-Li(1)-S(2) 91.41(2) × 2 Li(1)-S(1) 2.8573(6) × 2 S(1)-Li(1)-S(2) 86.74(2) × 2 Li(1)-Li(3) 2.782(3) × 2 S(1)-Li(1)-S(3) 88.13(2) × 2 Li(1)-Sn(1) 3.2777(6) × 2 S(1)-Li(1)-S(3) 91.87(2) × 2 Li(1)-Li(2) 3.350(3) × 2 S(1)-Li(1)-S(2) 93.26(2) × 2 S(1)-Li(1)-S(1) 180.0 S(2)-Li(1)-S(2) 180.0 S(3)-Li(1)-S(3) 180.0 Li(2)-S(2) 2.433(2) × 2 S(1)-Li(2)-S(3) 97.4(1) Li(2)-S(1) 2.587(4) S(1)-Li(2)-S(2) 106.6(1) × 2 Li(2)-S(3) 2.501(4) S(2)-Li(2)-S(2) 109.4(1) Li(2)-Sn(1) 3.118(4) S(2)-Li(2)-S(3) 117.5(1) × 2 Li(3)-S(2) 2.405(3) S(2)-Li(3)-S(3) 94.1(1) Li(3)-S(1) 2.438(3) S(1)-Li(3)-S(2) 100.4(1) Li(3)-S(3) 2.571(3) S(1)-Li(3)-S(3) 104.5(1) Li(3)-S(2) 2.511(3) S(2)-Li(3)-S(2) 111.6(1) S(1)-Li(3)-S(2) 118.9(1) S(2)-Li(3)-S(3) 122.6(1) Sn(1)-S(2) 2.3755(6) × 2 S(2)-Sn(1)-S(3) 105.78(2) × 2 Sn(1)-S(1) 2.3757(9) S(1)-Sn(1)-S(3) 106.07(2) Sn(1)-S(3) 2.4072(8) S(2)-Sn(1)-S(2) 111.57(2) S(2)-Sn(1)-S(1) 113.43(1) x 2 5 Symmetry equivalent positions for room temperature and 100K structures of Li4SnS4 Original Coordinates Data for the 296 K structure are reported using the conventions of the International Tables for Crystallography (Volume A) for expressing the asymmetric unit in space group 62, i.e. (0 ≤ x ≤ ½ ); (0 ≤ y ≤ ¼); (0 ≤ z ≤ 1). Initial data for the 100 K structure were taken from the file cm_3011315_si_002.cif, supplied as supporting material for Chem. Mater., 13 (2012) 47144721. by Kaib et al. 296 K x y z 100 K x y z Sn 0.0915 0.25 0.642 Sn 0.41339 0.25 0.63477 S1 0.083 0.25 0.2671 S1 0.4086 0.25 0.2626 S2 0.1608 0.0013 0.7835 S3 0.33254 0.49341 0.76654 S3 0.4323 0.25 0.7659 S2 0.57562 0.25 0.7641 Li1 0 0 0 Li4 0.465 0.517 -0.009 Li3 0.287 0.706 0.499 Li2 0.4085 0.25 0.1257 Li2 0.5696 0.25 0.162 Li3 0.1777 0.0035 0.1785 Li1 0.3396 0.4955 0.154 Common Origin Transformation To convert the two unit cells to a common origin, the following transformation was applied to the 100K data: x’ = (-x) + 0.5 6 296 K x y z 100 K x y z Sn 0.0915 0.25 0.642 Sn 0.08661 0.25 0.63477 S1 0.083 0.25 0.2671 S1 0.0914 0.25 0.2626 S2 0.1608 0.0013 0.7835 S3 0.16746 0.49341 0.76654 S3 0.4323 0.25 0.7659 S2 -0.07562 0.25 0.7641 Li1 0 0 0 Li4 0.035 0.517 -0.009 Li3 0.213 0.706 0.499 Li2 0.4085 0.25 0.1257 Li2 -0.0696 0.25 0.162 Li3 0.1777 0.0035 0.1785 Li1 0.1604 0.4955 0.154 Equivalent Positions To make comparison of the fractional coordinates easier, the atom positions in the 100 K solution that were not directly comparable were converted using the symmetry equivalent positions for space group 62. The specific transformations were as follows: Atom Site Symmetry Equivalent Position Applied Sn 4c (x,y,z) S1 4c (x,y,z) S3 8d (x, -y+ ½ , z) S2 4c (x + ½ , y, -z + ½ ) Li4 8d (-x + ½ , y + ½ , z + ½ ) Li3 8d (-x + ½ , y + ½ , z + ½ ) Li2 4c (x + ½ , y, -z + ½ ) Li1 8d (x, -y+ ½ , z) 7 Final Results 296K x y z 100K x y z Sn 0.0915 0.25 0.642 Sn 0.08661 0.25 0.63477 S1 0.083 0.25 0.2671 S1 0.0914 0.25 0.2626 S2 0.1608 0.0013 0.7835 S3 0.16746 0.00659 0.76654 S3 0.4323 0.25 0.7659 S2 0.42438 0.25 0.7359 Li1 0 0 0 Li4 0.465 0.017 0.491 Li3 0.287 0.206 0.999 Li2 0.4085 0.25 0.1257 Li2 0.4304 0.25 0.338 Li3 0.1777 0.0035 0.1785 Li1 0.1604 0.0045 0.154 In the 100K solution the Li3 and Li4 atoms each have 50% occupancy. The x and z fractional coordinates of Li(2) and Li(3) are highlighted in the table above, as these are the positions that show the greatest variation between structural solutions. 8 Comparison of fractional atomic coordinates a for structure reports of Li4GeS4. Matusushita & Kanatzidis 1 This work Atom siteb x y z atom siteb x y z Li(1) 4a 0.0000 0.0000 0.0000 Li(1)c 8d 0.0022 0.0143 0.0262 Li(2) 4c 0.4117 0.2500 0.1294 Li(2) 4c 0.4117 0.2500 0.1294d Li(3) 8d 0.1777 0.0000 0.1915 Li(3) 8d 0.1777 0.0000 0.1925 Ge(1) 4c 0.0890 0.2500 0.6485 Ge(1) 4c 0.0890 0.2500 0.6486 S(1) 4c 0.0862 0.2500 0.2906 S(1) 4c 0.0862 0.2500 0.2904 S(2) 8d 0.1567 0.0149 0.7787 S(2) 8d 0.1567 0.0149 0.7787d S(3) 4c 0.4391 0.2500 0.7308 S(3) 4c 0.4391 0.2500 0.7308 Kanno et al. 2 Murayama et al. 3 Li(3)c 8d 0.3050 0.2030 0.9820 Li(3) 4a 0.0000 0.0000 0.0000 Li(2) 4c 0.4240 0.2500 0.3290 Li(1) 4c 0.4127 0.2500 0.1315 Li(1) 8d 0.1650 0.0040 0.1750 Li(2) 8d 0.1768 0.0027 0.1997 Ge(1) 4c 0.0897 0.2500 0.6495 Ge(1) 4c 0.0892 0.2500 0.6494 S(2) 4c 0.0857 0.2500 0.2933 S(2) 4c 0.0871 0.2500 0.2966 S(1) 8d 0.1557 0.0135 0.7764 S(1) 8d 0.1572 0.0149 0.7758 S(3) 4c 0.4387 0.2500 0.7292 S(3) 4c 0.4390 0.2500 0.7315 a The coordinates reported in the previously published works have been transformed into equivalent positions, in order to compare directly to those obtained in this work. b The site is defined as the Wyckoff letter and multiplicity. c This lithium site is 50% occupied. Two typographical errors (wrong signs) were found in Kanatzidis’ publication and corrected here. d 9 [1] Y. Matusushita, M. G. Kanatzidis, Z. Naturforsch 53b (1998) 23-30. [2] R. Kanno, T. Hata, Y. Kawamoto, M. Irie, Solid State Ionics 130 (2000) 97 –104. [3] M. Murayama, R. Kanno, Y. Kawamoto, T. Kamiyama, Solid State Ionics 154-155 (2002) 789-794. 10 X-Ray Powder Diffraction Data for Li4GeS4 11 X-Ray Powder Diffraction Data for Li4SnS4 12