UNIT 1 Biochemistry Notes
advertisement
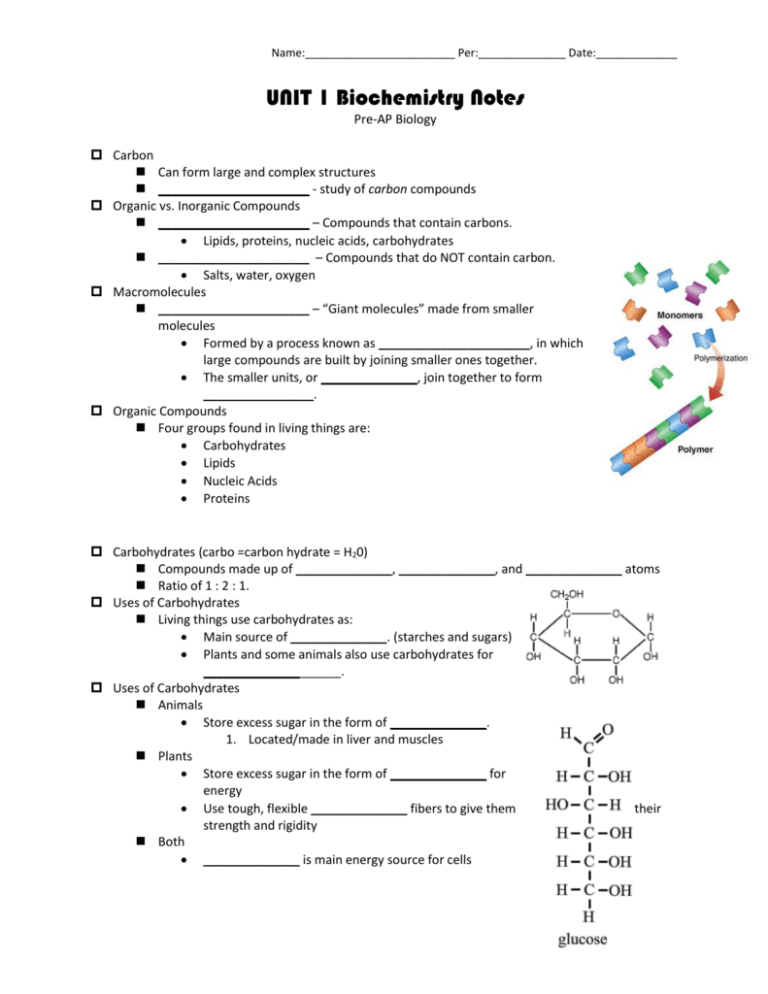
Name:________________________ Per:______________ Date:_____________ UNIT 1 Biochemistry Notes Pre-AP Biology Carbon Can form large and complex structures ______________________ - study of carbon compounds Organic vs. Inorganic Compounds ______________________ – Compounds that contain carbons. Lipids, proteins, nucleic acids, carbohydrates ______________________ – Compounds that do NOT contain carbon. Salts, water, oxygen Macromolecules ______________________ – “Giant molecules” made from smaller molecules Formed by a process known as ______________________, in which large compounds are built by joining smaller ones together. The smaller units, or ______________, join together to form ________________. Organic Compounds Four groups found in living things are: Carbohydrates Lipids Nucleic Acids Proteins Carbohydrates (carbo =carbon hydrate = H20) Compounds made up of ______________, ______________, and ______________ atoms Ratio of 1 : 2 : 1. Uses of Carbohydrates Living things use carbohydrates as: Main source of ______________. (starches and sugars) Plants and some animals also use carbohydrates for ____________________. Uses of Carbohydrates Animals Store excess sugar in the form of ______________. 1. Located/made in liver and muscles Plants Store excess sugar in the form of ______________ for energy Use tough, flexible ______________ fibers to give them their strength and rigidity Both ______________ is main energy source for cells Name:________________________ Per:______________ Date:_____________ Classification of Sugars ______________ – Single (simple) sugar molecules Examples: ______________, Galactose, Fructose ______________– Double sugar molecules Examples: 1. Glucose + Fructose = Sucrose 2. Glucose + Galactose = Lactose 3. Glucose + Glucose = Maltose ______________– More than two monosaccharide molecules Examples: Starch, Cellulose, Chitin, Glycogen Lipids Common categories of lipids are Fats Oils _________________ Functions: Can be used to store _____________ Some lipids are important parts of biological membranes and waterproof coverings Can serve as chemical messengers (steroids only) Generally not soluble in water Structure of Lipids Made mostly from _____________, _____________,and _____________atoms Usually has relatively small amounts of oxygen Glycerol molecule + 3 fatty acids (Triglycerides) Saturated and Unsaturated Lipids _____________________- If each carbon atom in a lipid's fatty acid chains is joined to another carbon atom by a _____________bond. “Saturated” means it has the maximum possible number of hydrogen atoms Solid at room temperature Examples – Cholesterol, butter, chocolate ____________________- If there is at least one carbon-carbon _____________bond in a fatty acid. Liquid at room temperature Examples - Corn oil, sesame oil, canola oil, and peanut oil Name:________________________ Per:______________ Date:_____________ Protein _____________- Macromolecules that contain nitrogen as well as carbon, hydrogen, and oxygen. Made up of chains of _____________folded into complex structures. Amino Acids - Compounds with an _____________ (−NH2) on one end and a _____________ (−COOH) on the other end. Amino Acids There are more than _______ different amino acids. Any amino acid may be joined to any other amino acid by bonding an amino group to a carboxyl group by dehydration synthesis. There are more than 20 different amino acids. What distinguishes one amino acid from another is the R-group (functional group) section of the molecule. Functions of Proteins Each protein has a specific role. Some proteins control the rate of reactions and regulate cell processes. _____________ Some are used to form bones and muscles and tissues. Structurally = collagen and keratin Others transport substances into or out of cells or help to fight disease. Antibodies Transport channels in the cell membrane Nucleic Acids ______________________ - Macromolecules containing hydrogen, oxygen, nitrogen, carbon, and phosphorus. Made up of repeating units called _____________ Each nucleotide contains: 5-Carbon Sugar Phosphate Group Nitrogenous Base Nucleic Acids Function: Store genetic information Transmit genetic information Two Kinds of Nucleic Acids: Ribonucleic acid (__________) Contains the sugar ribose Single stranded Deoxyribonucleic acid (___________) Contains the sugar deoxyribose Name:________________________ Per:______________ Date:_____________ Double stranded Dehydration Synthesis _____________ _____________ – A chemical reaction that builds up molecules by losing water molecules. Used to put together monomers to build polymers. The “dehydration” part is the removal of water The “synthesis” part is the joining of the two smaller compounds to create one larger one TRICK: There will always be one less water produced than the number of monomers joining together. Hydrolysis _____________ – The rupture of chemical bonds by the addition of water. Used to break down polymers into their monomers. Chemical Reactions _____________ _____________ - A process that changes one set of chemicals into another set of chemicals. Always involve the breaking of bonds in reactants and the formation of new bonds in products. Chemical Reactions and Water Molecules cannot react chemically unless they are in solution, so virtually all chemical reactions in the body depend upon water’s solvent properties. Most abundant inorganic compound in the body (accounts for 2/3 of body weight). Chemical Reactions _____________ - The elements or compounds that enter into a chemical reaction. _____________ - The elements or compounds produced by a chemical reaction. Na + Cl NaCl Energy in Reactions Because chemical reactions involve breaking and forming bonds, they involve changes in _____________. Will the chemical reaction occur? Chemical reactions that release energy often occur spontaneously. Energy is released in the form of heat, light, and sound. Chemical reactions that absorb energy will not occur without a source of energy. Every organism must have a source of energy to carry out necessary chemical reactions. Organisms and Energy Name:________________________ Per:______________ Date:_____________ Plants Get their energy by trapping and storing the energy from sunlight in energy-rich compounds. Animals Get their energy when they consume plants or other animals. Release the energy needed to grow tall, to breathe, or to think through the chemical reactions that occur when humans metabolize, or break down, digested food. Activation Energy _____________ _____________ - The energy that is needed to get a reaction started. The peak of each graph represents the energy needed for the reaction to go forward. The difference between this required energy and the energy of the reactants is the activation energy. Catalysts Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue and cells. _____________ - A substance that speeds up the rate of a chemical reaction by lowering a reaction’s activation energy. Enzymes Enzymes - Proteins that act as biological catalysts. Speed up chemical reactions that take place in cells. (by lowering activation energy) Very specific, generally catalyzing only one chemical reaction. Part of an enzyme's name is usually derived from the reaction it catalyzes. How Do Enzymes Work? _____________ - The reactants of enzyme-catalyzed reactions. The Enzyme-Substrate Complex Enzymes provide a site where reactants can be brought together to react. This site reduces the energy needed for reaction. Each protein has a specific, complex shape. _____________ – The site on the enzyme where substrates bind. The active site and the substrates have complementary shapes, which is often compared to __________________________ Regulation of Enzyme Activity Enzymes can be affected by any variable that influences a chemical reaction such as: pH Temperature Cells contain proteins that help to turn key enzymes “on” or “off”