Thermodynamics Homework: Ideal Gas Processes & Power Cycles
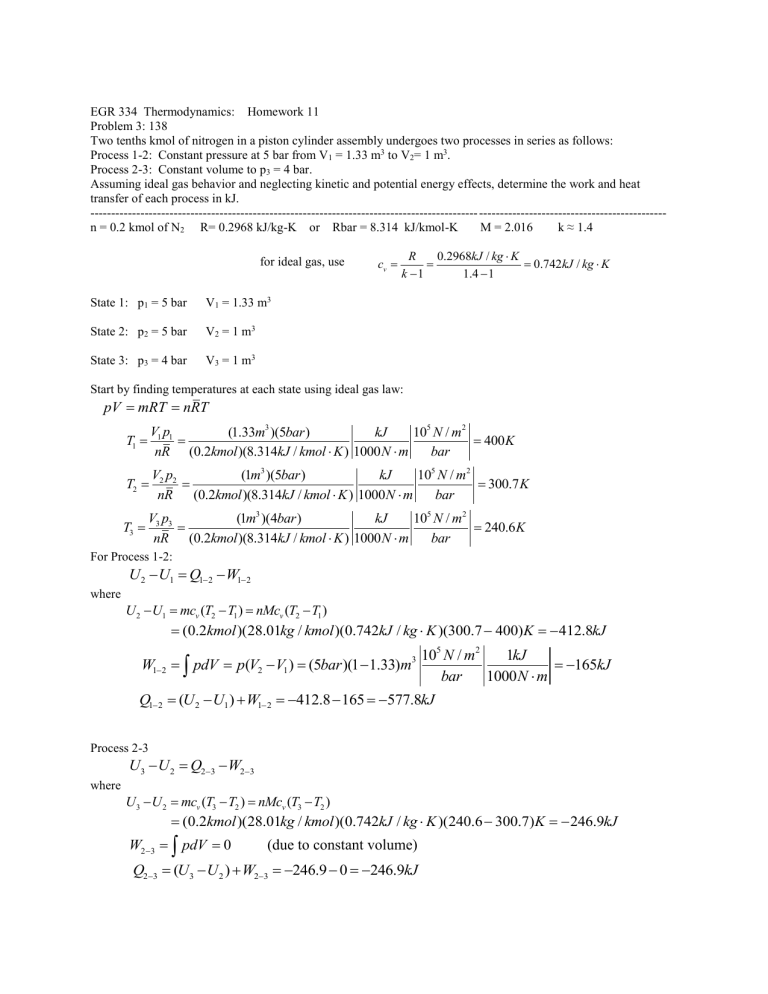
EGR 334 Thermodynamics: Homework 11
Problem 3: 138
Two tenths kmol of nitrogen in a piston cylinder assembly undergoes two processes in series as follows:
Process 1-2: Constant pressure at 5 bar from V
1
= 1.33 m 3 to V
2
= 1 m 3 .
Process 2-3: Constant volume to p
3
= 4 bar.
Assuming ideal gas behavior and neglecting kinetic and potential energy effects, determine the work and heat transfer of each process in kJ.
--------------------------------------------------------------------------------------------- --------------------------------------------- n = 0.2 kmol of N
2
R= 0.2968 kJ/kg-K or Rbar = 8.314 kJ/kmol-K M = 2.016 k ≈ 1.4
for ideal gas, use c v
k
R
1
0.2968
/
0.742
/
State 1: p
1
= 5 bar V
1
= 1.33 m 3
State 2: p
2
= 5 bar V
2
= 1 m 3
State 3: p
3
= 4 bar V
3
= 1 m 3
Start by finding temperatures at each state using ideal gas law: pV
mRT
nRT
T
1
V p
1 nR
(1.33
m
3
)(5 bar )
(0.2
kmol )(8.314
/
kJ 10
5
/
2
) 1000 N m bar
400 K
T
2
V p
2 nR
(1 m
3
)(5 bar )
(0.2
kmol )(8.314
/ kJ 10
5
N m
) 1000
bar
2
300.7
K
T
3
V p
3 nR
For Process 1-2:
where
U
2
U
1
Q
W
(1 m 3 )(4 bar )
(0.2
kmol )(8.314
/
kJ 10 5 N m 2
) 1000
bar
U
2
U
1
v
(
2
T
1
)
v
(
2
T
1
)
(0.2
kmol )(28.01
/ )(0.742
/
)(300.7
240.6
K
400) K
W
pdV
(
2
V
1
)
(5 bar
m
3
10
5
/
2 bar
1
1000 kJ
412.8
kJ
165 kJ
Q
( U
2
U
1
)
W
577.8
kJ
Process 2-3
U
3
U
2
Q
W where
U
3
U
2
v
(
3
T
2
)
v
(
3
T
2
(0.2
kmol )(28.01
/
W
pdV
0
)
)(0.742
/
(due to constant volume)
Q
( U
3
U
2
)
W
246.9
kJ
K
246.9
kJ
EGR 334 Thermodynamics: Homework 11
Problem 3: 142
One lb of oxygen undergoes a power cycle consisting of the following processes:
Process 1-2: Constant volume from p
1
= 20 psi , T
1
= 500 o R to T
2
= 820 o R
Process 2-3 Adiabatic expansion to V
3
= 1.432 V
2
.
Process 3-1: Constant pressure compression to State 1.
Sketch the cycle on a p-v diagram. Assume ideal gas behavior. Determine a) the pressure at state 2 in psi. b) the temperature at state 3 in deg R. c) the heat transfer and work (each in Btu) for all processes d) the thermal efficiency of the cycle.
--------------------------------------------------------------------------------------------------------------------------------------- m = 1 kg of O
2
R = 0.06206 Btu/lb-R c v
(T at 40 F) = 0.156 Btu/lb-R from Table A-20E
c v
(T at 300 F) = 0.164 Btu/lb-R
State 1: p
1
= 20 psi T
1
= 500 o R
State 2: V
1
= V
2
T
2
= 820 o R p
State 3: V
3
= 1.432 V
2 p
3
= 20 psi
Find V at State 1: pV
mRT
V
1
mRT
1
(1 lb m
)(0.06206
/ m
R p
1
(20 lb f
/ in 2 )
778.17
Btu f
1 ft
2
144 in 2
8.38
ft 3
2
1 v
At State 2: V
2
= 6.707 ft 3 p
2
mRT
V
2
2
(1 lb m
)(0.06206
(8.38
ft
/
3
)
At State 3: V
3
=1.432(8.38 ft 3 ) = 12.0 ft 3 m
R R 778.17
Btu f
1 ft 2
144 in
2
32.8
lb f
/ in
2
T
3
mR
lb f in
2 ft
3
(20 / )(12.0
) Btu
(1 lb m
)(0.06206
/ m
R ) 778.17
f
144 in
2
1 ft
2
715.6
R
For Process 1-2:
U
2
U
1
Q
W where (using c
U
2
U
W
Q
1
vave
= 0.16 Btu/lb m
-R)
v
(
2
T
1
)
(1 lb m
)(0.160
/ m
R pdV
0 (for constant volume)
( U
2
U
1
)
W
R
51.2
Btu
Btu 51.2 0 51.2
3
Process 2-3: Adiabatic expansion Q
2-3
= 0
U
3
U
2
Q
W where
U
3
U
2
v
(
3
T
2
(1 lb m
)
)(0.160
Btu lb m
R
R
16.7
Btu for adiabatic expansion: pV k constant
W
pdV
V
C k dV
p V
p V
2
1
k
(20 lb f
/ in
2
)(12.0
ft
3 lb f
/ in
2
)(8.385
ft
3
) 144 in
2
1 ft
2
Q
2 3
0
Note: work could also have been found using
W
2 3
Q
2 3
( U
3
U
2
)
For Process 3-1: (constant pressure)
U
1
U
3
Q
W where
U
1
U
3
v
(
1
T
3
)
(1 lb m
)(0.160
/ m
R
Btu
R
34.5
Btu
Btu
778.17
lb f
ft
16.6
Btu
W
pdV
(
1
V
3
)
(20 lb f
/ in
2 ft
3
144 in 2
1 ft
2
1 Btu
778.17
lb f
ft
13.4
Btu
Q
Summary:
( U
1
U
3
)
W
W
Q
1 2
51.2
Btu
47.9
Btu
0 W
Q
2 3
16.7
Btu W
0 Q
3 1
13.4
Btu
47.9
Btu
Thermal Efficiency:
W cycle
Q in
W
W
W
Q
51.2
0.0644
6.44%
EGR 334 Thermodynamics: Homework 11
Problem 3: 144
Air undergoes a polytropic process in a piston cylinder assembly from p
1
= 14.7 psi, T
1
= 70 deg F to p
2
= 100 psi.
Using IT, plot the work and heat transfer, each in Btu per lb of air for polytropic exponents ranging from 1.0 to 1.6.
Investigate the error in the heat transfer introduced by assuming constant c v
evaluated at 70 deg. F.
Discuss.
----------------------------------------------------------------------------------------------------------------------------- ----------
State 1: p
1
= 14.7 psi State 2: p
2
= 100 psi
T
1
= 70 deg F.= 530R
Examine how different n values from 1 to 1.6 affect work and heat transfer. Use c v
at 70 deg F
and R air
= 0.06855 Btu/lb-R.
Using the ideal gas law the volume of state 1 may be found (assume for 1 lb of mass): pV
mRT
V
1
mRT
1 p
1
(1 lb m
)(0.06855 / m
R )(530R) 778.17
1 ft
2
For polytropic process: pV n constant
Therefore:
14.7
lb f
/ in
2
Btu 144 in
2 p V
1 1 n p V
2 2 n
V
2
V
1
p p
1
2
1/ n
13.36
ft
3
V
2
13.365
14.7
1/ n
100
Again using ideal gas law the temperature of State 2 may be found:
T
2
p V
2 mR from 1 st Law of Thermo:
U Q W where
v
(
2
T
1
) and for n = 1
W
pdV W
V
C dV
p V
1 1 ln
V
V
1
2
for n ≠ 1
W
V
C n dV
C
( V
2
1
n
1
V
1
1
n
n
)
Therefore the Heat can be found by
Q U W
Now implement this in IT.
p V
2 2
p V
1 1
1
n
Solution using IT:
Explore with plot from n =1.1 to 1.6:
W and Q vs. n d U cv@70
and d U table
vs. n
Btu/lb m
Btu/lb m
Discussion: Notice that the ΔU curves diverge a little bit. This is because for one curve a Cv at 70 deg was used versus Table values found by IT. It is expected that the look up values would give more accurate results than using the Cv at one of the extreme temperatures. However using the Cv appears to be within acceptable error.
EGR 334 Thermodynamics: Homework 11
Problem 3: 147
Answer the following True or False:
----------------------------------------------------------------------------------------------------------------------------- ------------ a) If water initially a superheated vapor at 30 MPa, is cooled at constant pressure, the water eventually will become saturated vapor and then with sufficient additional cooling condensation to saturated liquid will occur.
The answer is False. This pressure is above the critical pressure, so there is not a transition which passes through a mixture phase.
----------------------------------------------------------------------------------------------------------------------------- ----------- b) A quasi-equilibrium process for which the pressure volume relation is describe by p/V n = constant, where n is a constant is called a polytropic process.
The answer is False: The definition for polytropic is pV n = constant not pV -n = constant
----------------------------------------------------------------------------------------------------------------------------------------- c) For simple compressible systems, the state principle indicates that the number of independent intensive thermodynamics properties required to fix an intensive state is two.
The answer is True.
----------------------------------------------------------------------------------------------------------------------------- ------------ d) For ammonia at 0.45 MPa and 50 deg C, the specific enthalpy is 1564.32 kJ/kg.
The answer is True. Consulting table A-13 at T=50 deg C, the sat pressure is 20.33 bar which is greater than the pressure given of 0.45 Pa = 4.5 bar. Therefore the substance is a vapor. Next consulting the superheated vapor table at 0.45 MPa at T = 40 deg h = 1540.64 and at T = 60, h = 1588.0 so by interpolation, the h at T = 50 gives
1564.32 kJ/kg.
--------------------------------------------------------------------------------------------------------------------- -------------------- e) For gases modeled as ideal gases, the value of the specific heat ration c v
/c p
is greater than 1.
The answer is false. The specific heat ratio, k is defined as c p
/c v
which will always be greater than 1.
-----------------------------------------------------------------------------------------------------------------------------------------









