general anesthetic
advertisement

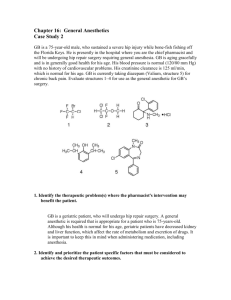
General Anesthetics General anesthetics are agents that cause loss of sensation, loss of consciousness and impair of motor function. History 1. Physically: by ice, tourniquets making ischemia or blow to the head. 2. Chemically: by ethanol or opium, nitrous oxide (laughing gas), diethyl ether (not used) due to its toxicity and (inflammable and explosive). 3. Now, ethers or halogenated hydrocarbons are used. General anesthesia employs multiple regimens to augment its action as barbiturates, benzodiazepines, skeletal muscle relaxants and also narcotic analgesics. Stages of Anesthesia: Stage I: Cortical stage or stage of analgesia and sleep (for surgeries which do not require skeletal muscle relaxation, e.g. tonsils removal). Stage II: Delirium stage (between unconsciousness and surgical anesthesia). Stage III: Surgical anesthesia; it is characterized by loss of excitement, muscle relaxation, unconsciousness, decreasing eye movement and regular respiration. Stage IV: Respiratory paralysis or medullary depression; characterized by depression of vital centres of the medulla and brain stem ⇒ respiratory and cardiac failure. This stage represents an overdose or toxic level that should be avoided (normally this stage should not be reached). 1 An ideal general anesthetic agent must produce loss of all sensation, analgesia and muscle relaxation. According to the route of administration, general anesthetics are classified into: 1. Inhalation Anesthetics. 2. Intravenous or Parentral Anesthetics. 3. Rectal (Basal) Anesthetics. 1. Inhalation Anesthetics: They are administered through the respiratory tract. They are either gases as N2O nitrous oxide gas, cyclopropane, ethyl chloride, or volatile liquids (more potent than gases), e.g. chloroform, ethers and other halogenated hydrocarbons. Cyclopropane CH2 H2C CH2 It is the most potent gaseous anesthetic agent (not currently used) characterized by rapid onset of action and fast recovery(highly inflammable). Synthesis: CH2 Br H2C CH2 Br CH2 Zn 2 H2C CH2 Ethyl Chloride CH3CH2 Cl Chloroethane Ethyl chloride is mainly used as local anesthetic by spraying to the skin and it also exerts general anesthetic properties when inhaled but its adverse side effects suppresses its use as general anesthetic (liver damage and cardiac arrhythmias). Synthesis: CH3CH2OH HCl ZnCl2 CH3CH2 Cl + H2O Nitrous Oxide N2O Nitrous oxide was used in the past for rapid induction of anesthesia in minor operations. It is prepared by heating ammonium nitrate. NH4NO3 Heat N2O Diethyl Ether CH3CH2 O CH2CH3 Diethyl oxide 3 + 2 H2O It is the most widely used general anesthetic. It is prepared by careful dehydration of ethanol with sulphuric acid at 135-140°C, or via Williamson reaction using ethyl bromide and sodium ethoxide. 2 CH3CH2OH H2SO4 CH3CH2 O CH2CH3 135-140C CH3CH2Br + CH3CH2ONa CH3CH2 O CH2CH3 Diethyl ether prepared by the first-method is usually accompanied by several by-products as ethylene and acetaldehyde. It is easily oxidized when exposed to air forming the highly explosive diethyl peroxide. It has prolonged induction time of anesthesia. Divinyl Ether CH2 CH O CH CH2 Divinyl oxide It has the advantage of rapid induction time (few seconds) compared to diethyl ether. Chloroform CHCl3 Trichloromethane 4 Chloroform was introduced as general anesthetic in 1847, its toxic effects on the liver and heart suppressed its medicinal use. Chloroform could be prepared as follows: 1. Controlled Chlorination of Methane CH4 2. + 3 Cl2 + 3 HCl Alkaline Hydrolysis of Chloral Hydrate Cl3CH(OH)2 3. CHCl3 NaOH CHCl3 + HCOONa + H2O Haloform Reaction using ethanol or acetone by the action of bleaching powder. CH3CH2OH + 2 CH3CHO + 2 CCl3CHO + CaOCl2 60C CH3CHO + Oxidation 6 CaOCl2 2 CCl3CHO + Ca(OH)2 2 CHCl3 + CaCl2 + H2O 3 Ca(OH)3 + 3 CaCl2 (HCOO)2Ca Anesthetic chloroform must be phosgene-free, which may be formed via photo-oxidation of chloroform. To avoid the formation of toxic phosgene, it must 5 be kept away from direct sunlight and 0.5% ethanol must be added to convert phosgene (if formed) to the non-toxic diethyl carbonate. 2 CHCl3 + Cl h O2 + 2O 2 HCl Cl OC2H5 COCl2 + 2 C2H5OH + O 2 HCl OC2H5 Halothane (Fluothane) F F Cl C CH F Br 1,1,1-Trifluoro-2-chloro-2-bromoethane. Halothane is four times as potent as diethyl ether, it is prepared from trichloroethylene as follows: Cl Cl C CH Cl HCl Cl Cl C CH2Cl Cl 3 HF F F Cl C CH F Br F Br2 F C F 6 CH2Cl In general, the rate of anesthesia induction (onset of action) depends on: a) Rate of respiration of the patient. b) Rate of delivery of the drug to the site of action (brain) which depend on the physicochemical properties of the drug (solubility in lipids and water). c) So if the drug water soluble ⇒ slow onset and long duration (long recovery period). d) If the drug is lipid soluble ⇒ rapid onset and short duration. SAR: 1. Number of carbons ⇒ lipid solubility. 2. Number of halogens ⇒ lipid solubility and H2O solubility (lipid > H2O). 3. Number of ethers ⇒ H2O solubility and lipid solubility (H2O > lipid). 2- Intravenous Anesthetics: The sodium salts of ultrashort acting barbiturates are the major members of this group, they may be administered intravenously in aqueous solutions for the induction of anesthesia. Respiratory depression is the main disadvantage of barbiturates; consequently, these agents are not used to maintain surgical anesthesia. Unconsciousness is produced within few seconds of intravenous injection, and the duration of action is about 30 minutes. In addition to the 7 ultrashort acting barbiturates, some miscellaneous compounds like Propfol and Ketamine hydrochloride are used for intravenous anesthesia. Thiopental Sodium (Pentothal Sodium) O N H3C S-Na+ H3C NH H3C O Sodium 5-ethyl-5-(1-methylbutyl)-2-thiobarbiturate It has ↑ lipid solubility due to branching and the presence of thio (S) group. It is the prototype of ultrashort-acting barbiturates. It has very short duration of action. Methohexital Sodium (Brevital Sodium) H3C O N O-Na+ N O CH3 Sodium 1-methyl-5-allyl-5-(1-methyl-2-pentynyl)barbiturate It has ↑ lipid solubility due to branching and the presence of CH3 groups. It has short duration of action. It is more potent than thiopental. 8 Ketamine Hydrochloride (Ketalar) O CH3 NH.HCl Cl 2-(o-Chlorophenyl)-2-methylaminocyclohexan hydrochloride It is the most widely used general anesthetic. It is very potent, rapid onset (rapid acting). Recovery is accompanied by “emergence delirium,” characterized by visual, auditory and confusional hallucinations, nightmares and dissociation from reality. Termination of action of ketamine occurs by its distribution from the brain into other tissues. Propofol CH3 OH H3C CH3 CH3 2,6-Diisopropylphenol 9 3- Rectal (Basal) Anesthetics: Tribromoethanol (Avertin) Br Br C CH2OH Br It is an example of this group. It is administered rectally as a solution in amylene hydrate. It is prepared by the reduction of bromal (tribromoacetaldehyde) with aluminium isopropoxide. Br Br Br C CHO + Al(OCH(CH3)2)3 H2O Br Br Br C CH2OH + Br O + Al(OH)3 Br Tribromoethanol Adjuvants to General Anesthesia: 1- Narcotic analgesic as morphine. 2- Sedatives as benzodiazepines e.g. midazolam (sleep-inducing). 3- Anticholinergic agents e.g. atropine. 4- Skeletal muscle relaxants, such as, succinyl choline. An ideal general anesthetic must have these previous four properties. 10