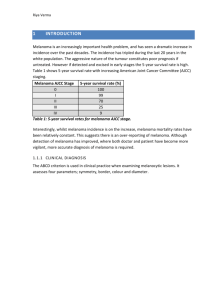
Pathophysiology of melanoma skin cancers - Ipswich
advertisement
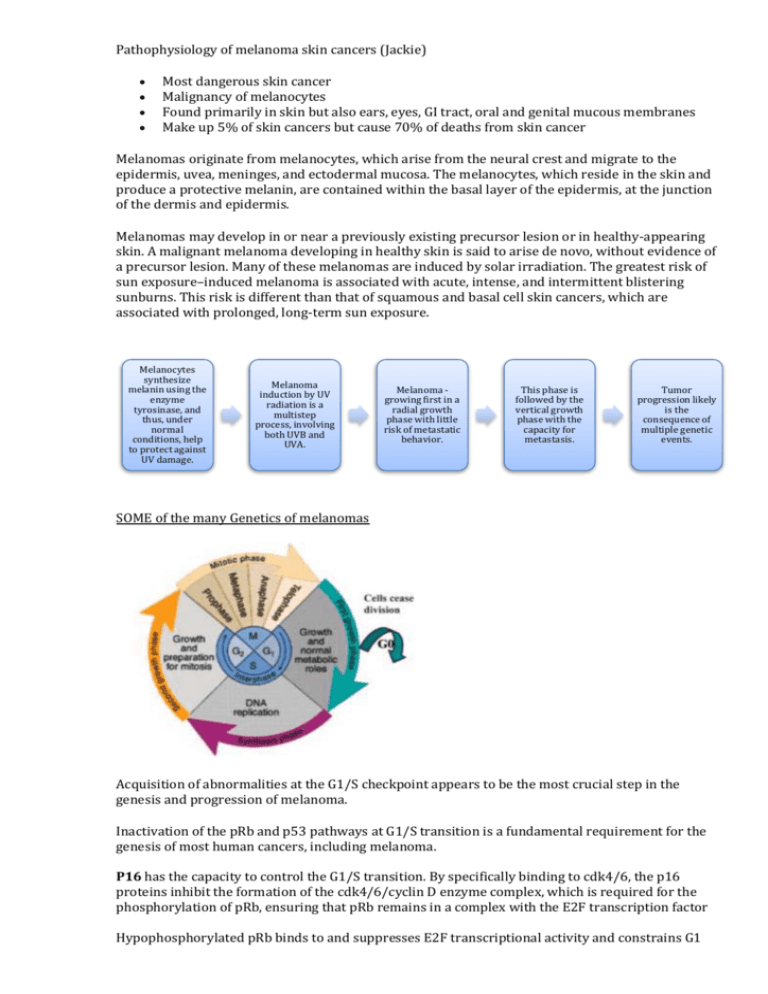
Pathophysiology of melanoma skin cancers (Jackie) Most dangerous skin cancer Malignancy of melanocytes Found primarily in skin but also ears, eyes, GI tract, oral and genital mucous membranes Make up 5% of skin cancers but cause 70% of deaths from skin cancer Melanomas originate from melanocytes, which arise from the neural crest and migrate to the epidermis, uvea, meninges, and ectodermal mucosa. The melanocytes, which reside in the skin and produce a protective melanin, are contained within the basal layer of the epidermis, at the junction of the dermis and epidermis. Melanomas may develop in or near a previously existing precursor lesion or in healthy-appearing skin. A malignant melanoma developing in healthy skin is said to arise de novo, without evidence of a precursor lesion. Many of these melanomas are induced by solar irradiation. The greatest risk of sun exposure–induced melanoma is associated with acute, intense, and intermittent blistering sunburns. This risk is different than that of squamous and basal cell skin cancers, which are associated with prolonged, long-term sun exposure. Melanocytes synthesize melanin using the enzyme tyrosinase, and thus, under normal conditions, help to protect against UV damage. Melanoma induction by UV radiation is a multistep process, involving both UVB and UVA. Melanoma growing first in a radial growth phase with little risk of metastatic behavior. This phase is followed by the vertical growth phase with the capacity for metastasis. Tumor progression likely is the consequence of multiple genetic events. SOME of the many Genetics of melanomas Acquisition of abnormalities at the G1/S checkpoint appears to be the most crucial step in the genesis and progression of melanoma. Inactivation of the pRb and p53 pathways at G1/S transition is a fundamental requirement for the genesis of most human cancers, including melanoma. P16 has the capacity to control the G1/S transition. By specifically binding to cdk4/6, the p16 proteins inhibit the formation of the cdk4/6/cyclin D enzyme complex, which is required for the phosphorylation of pRb, ensuring that pRb remains in a complex with the E2F transcription factor Hypophosphorylated pRb binds to and suppresses E2F transcriptional activity and constrains G1 exit, p16 expression results in cell cycle arrest. Loss of expression of p16 has been demonstrated in almost 50% of primary melanomas, it has been observed that loss of p16 expression correlates with increasing tumour thickness, higher mitotic rate, and increased proliferation rate. BRAF encodes a serine/threonine kinase downstream of RAS in the MAP kinase (MAPK) pathway. Mutated BRAF is responsible for the constitutive activation of the MAPK pathway, resulting in high levels of phosphorylated ERK that promote cell growth and proliferation. BRAF mutations have been found in approximately 40% to 80% of melanomas and nevi. The p53 tumour suppressor pathway often is inactivated in cancers; however, alterations of p53 itself rarely are seen in melanomas (0–25%). Melanoma cells have both radial and vertical growth phases. The radial growth phase encompasses horizontal growth in the epidermis. These lesions represent early-stage disease and may be curable with surgical excision. When the lesion enters the vertical growth phase, however, the repertoire of the many factors that influence tumorigenicity and metastasis changes as the tumour proliferates, enters the dermis, and acquires the capacity to metastasize. Tumour progression: Five stages of malignant transformation and tumor progression in melanocytes have been suggested: (1) benign melanocytic nevi; (2) atypical nevi; (3) primary malignant melanoma, radial growth phase; (4) primary malignant melanoma, vertical growth phase; and (5) metastatic malignant melanoma. With each successive step of tumorigenesis, a new clone of cells emerges with growth advantages over the surrounding tissue, resulting in “clonal expansion.” It has been postulated that a critical step in tumour progression of melanoma may be the transition from radial to vertical growth phases. The radial growth phase consists of primarily intraepidermal proliferation of melanoma cells, but also invasion of the papillary dermis by small numbers of cells that have gained a growth advantage. Types of melanomas: 1. Superficial spreading melanomas (SSM): 70% of cutaneous melanomas, often arises from a pigmented dysplastic nevus. 2. Nodular melanomas: 10-15% of melanomas, most symmetrical and uniform, dark brown or black in colour, radial growth may not be evident tumour advances quickly to vertical growth high risk lesion 3. Lentigo melanoma: found in sun-exposed areas, arise from lentigo maligna precursor lesion 4. Acral lentiginous melanoma: equal frequencies blacks and whites, occur on the palms, soles, and subungual areas, extremely aggressive and quickly advance to vertical growth phase 5. Mucosal lentiginous melanoma: develop from mucosal epithelium that lines the respiratory, GI and genitourinary tracts. Staging Breslow thickness is defined as the total vertical height of the melanoma, from the very top to the area of deepest penetration in to the skin. An instrument called an "ocular micrometer" is used to measure the thickness of the excised tumour. The Clark level refers to how deep the tumour has penetrated: Level I: confined to the epidermis, called "in situ" melanoma; 100% cure rate at this stage Level II: invasion of the papillary dermis Level III: filling of the papillary dermis, but no extension in to the reticular dermis Level IV: invasion of the reticular dermis Level V: invasion of the deep, subcutaneous tissue Prognosis Prognosis of a melanoma lesion can be predicted based on the following: the depth of invasion, presence or absence of ulceration and to nodal status at diagnosis. Malignant melanomas usually present at 2 extremes: at one end of the spectrum are patients with small skin lesions that are easily curable by surgical resection and at the other are patients with widely metastatic disease, in whom the therapeutic options are limited and prognosis is nil, with a median survival of only 6-9 months. References: Abeloff: Abeloff's Clinical Oncology, 4th ed. Access Medicine: Medical Oncology http://emedicine.medscape.com/article/280245-overview Li, W. et al. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology 38 (4), 297-301.