mec12497-sup-0001-AppendixS1
advertisement

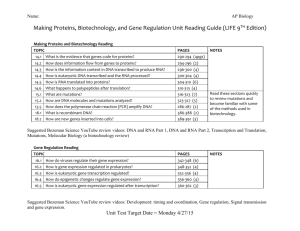
Supplementary Material Supplementary Materials and Methods Total RNA Isolation Each 2 mm3 liver sample was homogenized in TRIzol reagent as described by the manufacturer (Life Technologies) with additional care taken to minimize carry-over of RNAlater solution by blotting tissue samples onto a Kimwipe prior to addition to the TRIzol reagent. Mechanical disruption of each liver tissue utilized a 1.5 ml Safe-Lock tube (Eppendorf Canada, Mississauga, ON), 200 µl TRIzol, and disposable grinding pestle (Bio Basic Canada Inc., Markham, ON). Glycogen (20 µg) was added to each homogenate prior to the isopropanolmediated RNA precipitation step. Isolated tadpole liver total RNA was resuspended in 15 µl of diethyl pyrocarbonate (DEPC)-treated RNase-free water for the tadpoles at day 14 samples and in 30 µL DEPC-treated water for the Gosner stage 37 samples. The RNA yield of all samples was determined by spectrophotometry at 260 nm on a NanoDrop ND-1000 (Thermo Fisher Scientific Inc., Nepean, ON, Canada) with an average RNA concentration of ~400 ng/µl. All total RNA samples were subsequently stored at -70°C. Preparation of Total cDNA for Quantitative Real-Time Polymerase Chain Reaction (QPCR) and SMARTer cDNA for MAGEX DNA Microarray Analysis Tadpole liver total RNA (1 µg) from all samples was used with the High Capacity cDNA Reverse Transcription Kit as described by the manufacturer (Life Technologies) to produce total cDNA. Following the 2 h synthesis reaction, all cDNA samples were diluted 20-fold prior to QPCR amplification and stored at -20oC until further use. Hepatic RNA samples from six Gosner stage 37 animals originating from each of four islands representing the permanent and temporary pool island populations (Ålgrundet, SävarTärnögern, Öster Hällskär, and Stora Fjäderägg) reared under constant and decreasing water conditions were selected for microarray analysis (n=48). The MAGEX DNA microarray was comprised of 450-550 bp cDNA fragments representing 497 gene transcripts derived primarily from Xenopus laevis with additional cDNA fragments from Rana catesbeiana and Rana pipiens 1 open reading frames (Veldhoen et al., 2006). These cDNA fragments were printed on Erie C28 aminosilane (Thermo Fisher Scientific) coated slides in triplicate by the Jack Bell Prostate Cancer Research Centre (Vancouver, BC, Canada). Sample integrity was confirmed by electrophoresis of 2-3 µg total RNA on a 1% agarose gel followed by visualization with ethidium bromide staining and confirmation of the presence of strong equivalent 28S and 18S rRNA bands with no evidence of RNA degradation. The 48 RNA samples were converted to cDNA and labeled using the SMARTer PCR cDNA Synthesis Kit as per the manufacturer’s recommended protocol (Clontech Laboratories Inc., Mountain View, CA, USA). Each SMARTer cDNA was amplified on a Bio-Rad MyCycler for 24 cycles using the Advantage 2 PCR Kit (Clontech) as per the manufacturer’s protocol. Amplified cDNA was isolated using the GeneJET PCR Purification Kit as described by the manufacturer (Thermo Fisher Scientific) and eluted with 32 µl dH2O. The DecaLabel DNA Labeling Kit and protocol (Thermo Fisher) was used to label 500 ng of each SMARTer cDNA in a 50 µL reaction containing 2 µl of 1 mM Cy3-dCTP that was incubated at 37 °C for 15 min for both the pulse and chase (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). A universal DNA reference (UDR) comprising a composite of cDNA amplicons representing all gene sequences on the MAGEX DNA microarray was similarly labeled in a DecaLabel reaction containing 450 ng cDNA and 1 µl of 1 mM Cy5-dCTP (GE Healthcare). Deionized water was added to each completed reaction to a final volume of 100 µl and labeled DNA purified using the GeneJET PCR Purification Kit and eluted in 50 µl dH2O. Labeled DNA was concentrated by sequential addition of 20 µg glycogen, 5 µl 3M Na acetate (pH 5.2), and 150 µl 100% ethanol followed by incubation at -20 °C for one hour. DNA precipitate was collected by centrifugation at 12000 x g for 15 min and washed with 300 µl 75% ethanol followed by centrifugation for 3 min at 12000 x g. Each Cy3 labeled cDNA pellet was dried and resuspended in 7 µl dH2O while dried Cy5 labeled UDR was resuspended in 30 µl dH2O. Average Cy fluor incorporation amounts were 1015 pmol/µl Cy3-cDNA and 1.5 pmol/µl Cy5-UDR. MAGEX DNA Microarray Analysis of Hepatic mRNA Levels Samples representing the different islands and water treatment types were randomized prior to microarray analysis to prevent technical bias. Each MAGEX DNA microarray slide was 2 briefly rinsed in a plastic Coplin jar containing WB2 buffer (0.1xSSC, 0.1% w/v SDS) and subsequently incubated with 90 ml of PB1 buffer (5x SSC, 0.2% w/v BSA, and 0.2% w/v SDS) at 48°C for 15 min. Treated slides were rinsed twice in a plastic Coplin jar filled with dH2O and then washed with isopropanol. Each slide was dried by centrifugation in a 50ml Falcon tube for 3 min at 200 x g. Dried coverslips prerinsed successively with dH2O and 100% ethanol were placed over each DNA array region. Cy-labeled UDR (1 µl) was mixed with each Cy-labeled cDNA (5.5 µl) and incubated at 95 °C for 3 min followed by cooling on ice and a brief centrifugation to collect the denatured material. For each sample, a 33 µl hybridization solution was prepared that contained 5x SSC, 25% v/v deionized formamide, 0.02% w/v bovine serum albumin, 1 µg A45 oligonucleotide, 0.1% w/v SDS, 5% w/v dextran, and 6.5 µl denatured Cylabeled DNA and warmed briefly at 37 °C prior to application onto the covered MAGEX DNA microarray. MAGEX DNA microarray slides were placed in a slide rack and 300 µl of dH2O was added to the humidity filter prior to chamber assembly. Hybridization was done for 18 h at 42 °C in a Hybex Microsample Incubator (SciGene, Sunnyvale, CA, USA). All wash buffers were prewarmed at 48 °C prior to use. Following hybridization, coverslips were removed and each slide washed for 5 min with WB1 buffer (1x SSC and 0.1% w/v SDS). Two subsequent washes were performed with WB2 buffer followed by a wash with WB3 buffer (0.01x SSC and 0.1% w/v SDS). Slides were dried by centrifugation for 3 min at 200 x g and stored in the dark in a Scienceware autodessicator (Bel-Art, Pequannock, NJ, USA). MAGEX DNA microarray image data were collected at 5 µm from each slide using a Scan Array Express (Packard Bioscience Co., Meriden, CT, USA) as recommended by the manufacturer and at a PMT gain of 70% for the Cy5 channel and 80% for the Cy3 channel. The laser level was set at 90% for both channels. Gene expression data was obtained from MAGEX DNA microarray images using ImaGene Version 9 (BioDiscovery Inc., El Segundo, CA, USA) as per the manufacturer’s protocol. Statistical Analysis of the MAGEX DNA Microarray Data The background signal for each MAGEX DNA microarray was determined as the sum of the median and one robust standard deviation of buffer-only and empty spotted positions interspersed throughout each array. The range of background signals was 241-353 and the 3 maximum background across arrays was selected as a universal floor value to minimize false positives due to low signal intensities. Selection of gene spot signals for normalization factor determination was performed by identifying gene transcripts with robust signals (>500) and no missing values across all of the microarrays and evaluating the signals for island or treatment effects (Kruskal Wallis test, SYSTAT 13, Systat Software Inc., Chicago, IL, USA). A total of 27 gene spots displayed both robust signal and invariance across the 48 arrays (Supplementary Table S1). Normalization factors were generated for each array gene data set by dividing the geometric mean of the 27 invariant gene spots from the array that displayed the greatest overall signal levels with the geometric mean for the same 27 genes derived from each individual array. This resulted in normalization factors between 1.00 and 4.19. Normalization factors were then used to adjust the global signal intensity present across the array data sets and the universal floor was subsequently applied. Median gene transcript signal values for each array were then calculated from the triplicate gene spot values. Effective normalization across the 48 MAGEX DNA microarrays was confirmed through intraclass correlation analysis of median gene transcript signal values using PASW Version 18.0 software (SPSS Inc., Chicago, IL, USA). Correlates of 0.979-0.986 for average measures (consistency) and 0.979-0.986 for average measures (absolute agreement) were obtained. Significant differences in gene transcript abundance were identified using the non-parametric Kruskal-Wallis test and pair-wise comparisons of treatment grouped data using the Mann-Whitney U test in SYSTAT 13. The same program was used to calculate Spearman’s rho correlation coefficients. Significance was Bonferroni corrected for multiple tests where appropriate with P<0.05. Gene ontologies were examined from the significant results with DAVID using the entire MAGEX DNA microarray as background (Huang et al. 2009a, 2009b). The microarray data set has been submitted to the Gene Expression Omnibus (GEO number GSE42274). Targeted Cloning of Gene Sequences and Quantitative Real-Time Polymerase Chain Reaction (QPCR) Primer Design All cloning and QPCR DNA oligonucleotide primers were designed using Primer Premier Software version 5 (Premier Biosoft International, Palo Alto, CA, USA) and supplied by 4 Integrated DNA Technologies (Coralville, IA, USA). Isolation of R. temporaria cDNA sequence for each target gene was carried out using cloning PCR primers designed using similar cDNA sequence information from other anuran species and were deposited in the National Center for Biotechnology Information (NCBI) GenBank Database (http://www.ncbi.nlm.nih.gov/genbank/). DNA amplification reactions were performed on a MyCycler (Bio-Rad Laboratories) using 2 Units Maxima HotStart Taq DNA polymerase (Thermo Fisher Scientific) as per the manufacturer’s recommended protocol. Each 25 µl reaction also contained 3 µl of a 20-fold diluted tail and brain combined cDNA and 20 pmol of each cloning primer. The thermocycle program consisted of an initial denaturation step of 94°C (5 min) followed by 45 cycles of 94°C (15 sec), 52°C (30 sec), and 72°C (60 sec), and a final elongation step of 72°C (10 min). Amplified DNA products were separated by electrophoresis on a 1.5% agarose gel, visualized with ethidium bromide staining, and DNA amplicons of the correct predicted size were excised from the gel. DNA was isolated from the gel matrix through centrifugation for 10 min at 10,000 x g in an Ultrafree-DA column (Millipore Corp., Bedford, MA, USA). Purified DNA amplicons (2 µl) were blunt-ended and ligated into plasmid vector using the CloneJET PCR Cloning Kit as per the manufacturer’s recommended protocol (Thermo Fisher Scientific). A portion of each ligation reaction (3 µl) was used to transform 25 µl of high-efficiency chemically competent NEB5α E. coli cells as per the manufacturer’s recommended protocol (New England Biolabs Inc., Pickering, ON, Canada). Competent cells mixed with ligated DNA were incubated 30 min on ice followed by 30 sec at 42°C and 5 min on ice. SOC media (500 µl) was added and the cells were allowed to recover for one hour at 37°C with shaking. E. coli transformed with ligated plasmid DNA were selected for by plating 100 µl of each transformation solution onto LB agar plates containing 100 µg/ml ampicillin and 0.5 mM isopropyl-beta-D-thiogalactopyranoside (IPTG). Plates were incubated overnight at 37°C. Select bacterial colonies were transferred to a master plate and those containing the correct recombinant plasmid identified by colony PCR in a 15 µl DNA amplification reaction containing 1 Unit of Maxima Hot Start Taq DNA polymerase (Thermo Fisher Scientific) and 1.5 pmol each of forward and reverse vector-specific primers. The MyCycler thermocyle program consisted of an initial denaturation step of 94°C (5 min) followed by 35 cycles of 94°C (15 sec), 55°C (30 sec), and 72°C (45 sec), and a final elongation step of 72°C (10 min). Amplified DNA products were separated by electrophoresis on a 1.5% 5 agarose gel and visualized with ethidium bromide staining. Positive bacterial colonies were used to inoculate 5 ml of LB broth containing 100 µg/ml ampicillin and cultures were grown at 37°C overnight with shaking at 250 rpm. Plasmid DNA was recovered from the cell cultures using a GeneJet Plasmid Miniprep Kit according to the manufacturer’s instructions (Thermo Fisher Scientific). DNA sequencing was carried out by Eurofins MWG Operon (Huntsville, AL, USA). The identity of each R. temporaria cDNA was confirmed by BLASTn DNA alignment analyses (http://www.ncbi.nlm.nih.gov/BLAST/) and subsequently deposited in the NCBI GenBank Database under accession numbers JX997954 (ribosomal protein L8; rpl8), JX997955 (thyroid hormone receptor ; thra), JX997956 (thyroid hormone receptor ; thrb), JX997959 (carbamoyl phosphate synthetase; cps1), JX997964 (ornithine transcarbamylase; otc), and JX997961 (catalase; cat). QPCR primer sets directed towards R. temporaria gene targets were designed and evaluated through an extensive quality control assessment on a MX3005P RealTime PCR System (Agilent Technologies Canada Inc, Mississauga, ON, Canada). Primer pairs that met all quality criteria, including verification of appropriate amplicon targeting and satisfying the requirements for use in the comparative Ct method (see Veldhoen et al., 2009 for details) were used for generation of mRNA abundance data (Supplementary Table S2). Real-time Quantitative PCR (QPCR) Assessment of Hepatic mRNA Levels Samples representing all six islands and water treatment types were randomized prior to performing the QPCR assay and the samples were run blind on the 20-fold diluted total cDNA samples to minimize experimental bias. The 15 µl QPCR reaction contained 10 mM Tris HCl pH 8.2 at 20°C, 50 mM KCl, 3 mM MgCl2, 0.01% Tween 20, 0.8% glycerol, 40,000-fold dilution of SYBR Green I (Life Technologies), 200 µM dNTPs (Thermo Fisher Scientific), 83.3 nM ROX reference dye (Life Technologies), 5 pmol of each primer, 2 µl of 20-fold diluted total cDNA, and 1 Unit of Maxima Hot Start Taq DNA polymerase (Thermo Fisher Scientific). The thermocycle program for each transcript included an initial activation step at 95°C (9 min); 40 cycles of 95°C denaturation (15 sec), 60°C annealing (30 sec), and 72°C extension (45 sec). Specificity of target amplification was measured by the inclusion of reactions lacking cDNA (no DNA template control) and by subjecting completed runs to melting curve analysis. For each gene target, inter-run variation in reagent performance was assessed by the presence of a 6 standard control amplification reaction that included pooled R. temporaria liver cDNA. An overall variation of cycle threshold (Ct) value ≤1 was considered acceptable. For all genes investigated, quadruplicate reactions were performed on each individual cDNA sample and Ct data obtained were evaluated using the comparative Ct method (Ct) with data normalized against a ribosomal protein L8 (rpl8) transcript Ct values to correct for variation in cDNA input as described previously (Supplementary Figure S1; Veldhoen et al, 2006). Statistical Analysis for the QPCR Data More extensive analyses using ANOVA and regressions were possible with QPCR data generated from the complete set of animals derived from all six island populations. The raw data used in these analyses are provided in Supplementary Tables S5 and S6. Initially we analysed the data using pool drying category (permanent pools: Stora Fjäderägg and Öster Hällskär, temporary pools: Ålgrundet and Sävar-Tärnögern, and intermediate pools: Bredskär and Petlandsskär) and phenotypic plasticity categories (high phenotypic plasticity: Petlandsskär and Bredskär) and low phenotypic plasticity: Stora Fjäderägg, Öster Hällskär, Ålgrundet and SävarTärnögern) as factors. However, there were no significant main effects using these factors and therefore we focussed on comparisons among islands. Gene transcript abundance levels were analyzed with two-way ANOVAs using the water treatment (constant and decreasing water) and island population as factors, and we did these analyses at day 14 of development and at Gosner stage 37. This allowed us to examine if transcript abundance differed between treatments and island populations. We treated islands as fixed factor because islands were chosen a priori based on their characteristics with regard to tadpole development and pool drying. If the interaction term was non-significant (P > 0.10) we re-ran the ANOVA without the interaction. Development stage at day 14 and time to development and weight at Gosner stage 37 were analyzed with similar statistical approaches as described above for the gene transcripts. At Gosner stage 42 we examined weight and development time to reach this stage for three of the islands. We measured three full-siblings from each female, and therefore we analyzed development to and size at Gosner stage 42 in a mixed model, incorporating the originating female as a random factor using the package lme4 in R 2.14.0 (R Development Core 7 Team 2011). The significance of the fixed effects was assessed using Wald χ2-tests implemented in the car library. Regression approaches were used to uncover possible relationships between gene transcripts responsible for the plastic effect in tadpole development and the constitutive effect on development. More specifically, we asked if a tadpole that constitutively develops faster in constant water conditions (e.g. from a temporary pool island population) is defined by the same transcript profile as tadpoles undergoing fast development induced by decreasing the water level. For this we used gene transcript abundance at day 14. In the first comparison, we examined the relationship between difference in developmental stage attained between the two treatments and the difference in gene transcript abundance between the two treatments, using a linear regression approach. Hence we calculated the difference in development stage for each sib-ship between the two treatments and the difference in gene transcript abundance for each sib-ship and then we applied regression with these two values against each other using island population as a factor. A significant relationship would suggest that the gene transcript is linked to the degree of plasticity in development time. In a second analysis, we applied regression to development stage under constant water treatment against gene transcript abundance for each individual using island population as factor. A significant relationship would suggest that the genes are linked to a constitutive response. Since Gosner stage is ordered categorical data, the relationship between development stage and gene transcript abundance was investigated using ordinal regression (also known as the proportional odds model), implemented using the package ordinal in the statistical software R 2.15.3 (R Development Core Team 2011). We tested the influence of population, gene transcript level and their interaction on development stage by comparison between models of increasing complexity using log likelihood-ratio tests against a chi-square distribution with degrees of freedom equal to the difference in df between the compared models (Crawley 2007). In addition, a hierarchical cluster analyses were performed on the log2-transformed median gene transcript abundance (n=6-10 animals/island) using Cluster 3.0 (Eisenlab, Stanford University, Stanford, CA, USA) with correlations centered and average linkage clustering. The output was visualized with Java TreeView version 1.16r2 (jtreeview.sourceforge.net). 8 References Crawley MJ (2007) The R book. Wiley, Chichester, England; Hoboken, N.J. Huang DW, Sherman BT, Lempicki RA (2009a) Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protocols, 4: 44-57. Huang DW, Sherman BT, Lempicki RA (2009b) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research, 37, 1-13. R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Veldhoen, N, Skirrow, RC, Ji, L, Domanski, D, Bonfield, ER, Bailey, CM and CC Helbing (2006) Use of heterologous cDNA arrays and organ culture in the detection of thyroid hormone-dependent responses in sentinel frog species. Comparative Biochemistry and Physiology D, 1, 187-199. Veldhoen, N Lowe, C Davis, C Mazumder A, Helbing CC (2009) Gene expression profiling in the deep water horse mussel Modiolus modiolus (L.) located near a marine municipal wastewater outfall. Aquatic Toxicology, 93, 116-124. 9