Word Version
advertisement
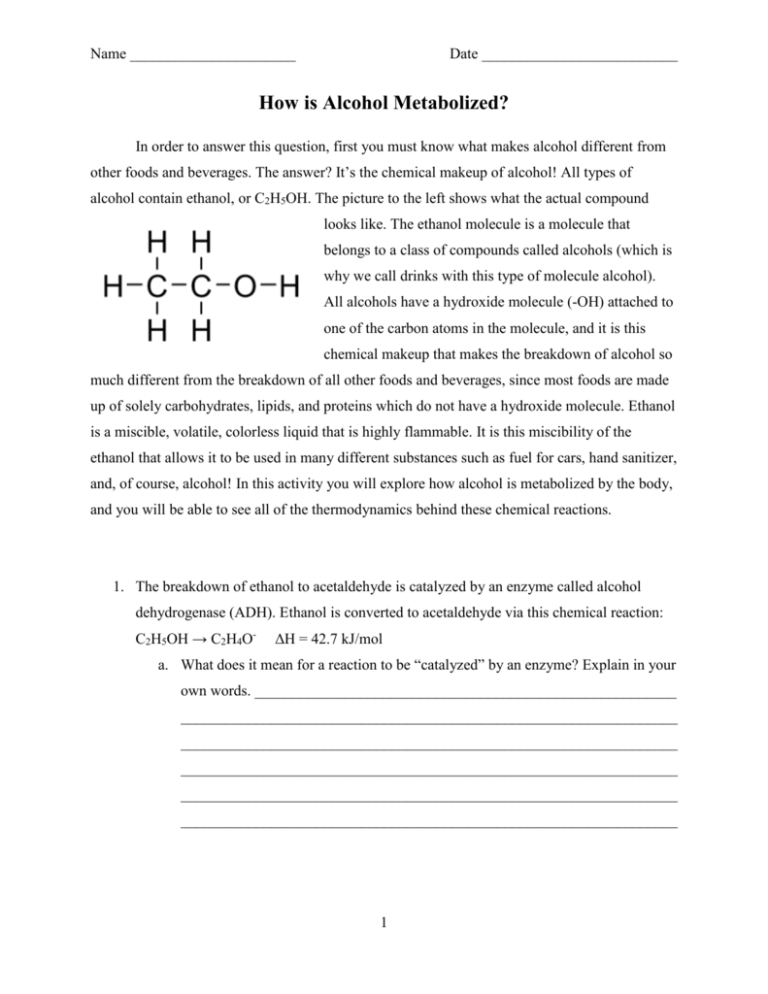
Name ______________________ Date __________________________ How is Alcohol Metabolized? In order to answer this question, first you must know what makes alcohol different from other foods and beverages. The answer? It’s the chemical makeup of alcohol! All types of alcohol contain ethanol, or C2H5OH. The picture to the left shows what the actual compound looks like. The ethanol molecule is a molecule that belongs to a class of compounds called alcohols (which is why we call drinks with this type of molecule alcohol). All alcohols have a hydroxide molecule (-OH) attached to one of the carbon atoms in the molecule, and it is this chemical makeup that makes the breakdown of alcohol so much different from the breakdown of all other foods and beverages, since most foods are made up of solely carbohydrates, lipids, and proteins which do not have a hydroxide molecule. Ethanol is a miscible, volatile, colorless liquid that is highly flammable. It is this miscibility of the ethanol that allows it to be used in many different substances such as fuel for cars, hand sanitizer, and, of course, alcohol! In this activity you will explore how alcohol is metabolized by the body, and you will be able to see all of the thermodynamics behind these chemical reactions. 1. The breakdown of ethanol to acetaldehyde is catalyzed by an enzyme called alcohol dehydrogenase (ADH). Ethanol is converted to acetaldehyde via this chemical reaction: C2H5OH → C2H4O- ΔH = 42.7 kJ/mol a. What does it mean for a reaction to be “catalyzed” by an enzyme? Explain in your own words. ________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 1 b. Based on the enthalpy (ΔH) of this reaction, is this reaction endothermic or exothermic? In your own words, describe what this means. __________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 2. After the ADH converts ethanol to acetaldehyde, another enzyme called acetaldehyde dehydrogenase (ALDH) further breaks down the acetaldehyde into acetate as shown here: C2H4O→C2H3O- ΔH = -215.1 kJ/mol. a. Based on the enthalpy (ΔH) of this reaction, is this reaction endothermic or exothermic? In your own words, describe what this means.__________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ 3. The next step in the breakdown of alcohol is for the body to break down acetic acid into carbon dioxide (CO2) and water (H2O) via the Krebs cycle. The overall reaction that occurs in the Krebs cycle is this: CH3COOH 2CO2 + 3H2O ΔH = -1325.3 kJ/mol a. Based on the enthalpy (ΔH) of this reaction, is this reaction endothermic or exothermic? _______________________________________________________ __________________________________________________________________ 2 4. Calculate the overall enthalpy of the breakdown of alcohol. Make sure to include all three steps and show your work! ΔH = ____________ a. Is the overall breakdown of alcohol exothermic or endothermic? ______________ __________________________________________________________________ b. The first step in the breakdown of ethanol to acetaldehyde is endothermic. Give an explanation for why this reaction actually occurs. _______________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ c. ATP is a biological molecule that is created when food is metabolized and is broken down by the body to be used as energy. If the creation of ATP requires 30.5 kJ/mol, how many moles of ATP molecules can be created per mole of ethanol breakdown? # ATP moles: ___________ 3 d. Whenever an ATP molecule is broken down into ADP, it releases 7 Calories/mol. Based on the number of moles ATP molecules you calculated in the previous question, how many Calories do you gain from the breakdown of 1 mole of ethanol? # Calories: ____________ e. A 1.5 oz. shot of 80 proof (40%) alcohol contains 0.6 oz. of ethanol, or 17.01 g of ethanol. The molecular weight of ethanol is 46.07 g/mol. How many moles of ethanol are in one 1.5 oz. shot? How many Calories do you gain from the breakdown of one 1.5 oz. shot of 80 proof alcohol? Moles ethanol: ___________ # Calories: ______________ 4 5. One pound of fat is equivalent to 3500 Calories. How many 1.5 oz. shots of 40% alcohol would a person have to drink in order to gain one pound? # shots:___________ 6. Many people who drink a lot of alcohol gain weight. Based on the possible number of Calories you calculated above, explain why this happens. _________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 7. The Krebs cycle breaks down acetic acid at a steady rate, while the rate of breakdown of ethanol and acetaldehyde are based on the number of ADH and ALDH enzymes in the body. For each of the cases below, determine what the rate limiting step is, and match each case to the effect it would have: high BAC level, high level of alcohol toxicity, no adverse effects. a. Describe, in your own words, what a rate limiting step is. ___________________ __________________________________________________________________ __________________________________________________________________ b. Very few ADH enzymes, many ALDH enzymes. __________________________________________________________________ __________________________________________________________________ c. Many ADH enzymes, very few ALDH enzymes. __________________________________________________________________ __________________________________________________________________ d. Many ADH enzymes, many ALDH enzymes. __________________________________________________________________ __________________________________________________________________ 5









