NAME: Ch 8,9 and Introduction to Chapter 10 Changes in Matter p
advertisement

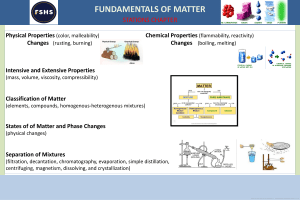
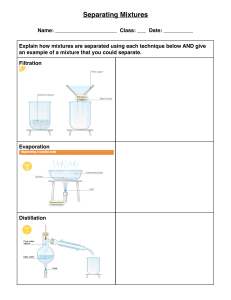
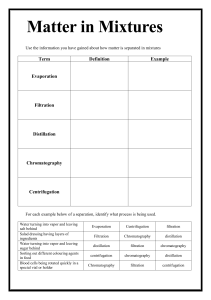
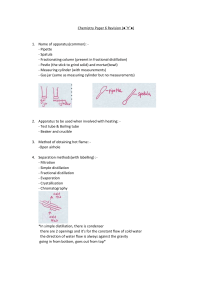
NAME: ____________________ Ch 8,9 and Introduction to Chapter 10 Changes in Matter p. 50 flexbook. Complete assignment and turn in, by end of period for full credit. Lesson Goals: Describe how to separate the different types of mixtures Propose how to separate different types of mixtures Identify chemical properties Know differences between chemical and physical changes Know how to identify if a chemical change is taking place ___Vocabulary for this section (just read): Chemical change, physical change, chromatography, distillation, evaporation, filtration, chemical property ___Review: 1. How are chemical and physical properties different? 2. Give two examples each of physical and chemical properties. 3. How are elements and compounds alike? And different? 4. How are pure substances alike? And different? (create a graphic organizer!) Changes in matter (fill in the blanks): ________________ changes alter the composition of a substance. ___________________result in a change in form of a substance, but not chemical composition. How to separate mixtures: Describe what chromatography is and how to do it. What is a practical example of chromatography? ------------------------------------------------------------------------Describe what distillation is. How does distillation work? How is distillation used? --------------------------------------------------------------------------Describe what evaporation is. How could it be used in a lab situation? ----------------------------------------------------------------------------Describe filtration and for what it can be used. ------------------------------------------------------------------------------------------------------------------------- Next page- Chemical changes are also known as ______________________________________. Three examples of chemical changes include: 1. 2. 3. List the visual clues you can use to determine if a chemical change has taken place: Write some common chemical reactions in which you can see a chemical change occurring. Which one did the class recently observe take place? All chemical changes involve a _____________ of energy. Sometimes energy is ____________ and sometimes it is _______________. ____Write a two paragraph summary about this section here: