outcomes assessment for academic programs
advertisement

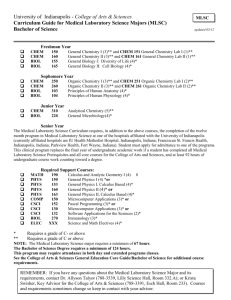
ACADEMIC PROGRAM ASSESSMENT OF STUDENT LEARNING Phase 1 Report, 2013-14 Submission Date______10/31/2013________ College____Arts and Sciences_______________ Department __Chemistry and Biochemistry_ Program___Chemistry & Biochemistry____ Degree Granted_BA (Chem); BS (Chem & Biochem) If multiple programs are included, please list additional programs here (graduate & undergraduate must be separate): __________________________________________ __________________________________________ Check one: Undergraduate ______X ____ Graduate _____________ Person Completing Report __David E. Smith_________________ Office Phone ___646-5210______________ Title _______Associate Professor_______________________________ E-Mail __davsmith@nmsu.edu___ Department Head (if different from person completing report)__William Quintana_____________________ Office Phone___646-5877_____________________________________ E-Mail _wquintan@nmsu.edu___________ Assessment will be implemented: Fall __2013_____ (year) Spring ___________ (year) External Accrediting Agency (if applicable)__________N/A_____________________________________________________ Date of last accreditation site visit_________________ Date of next accreditation site visit_______________ ***Attach copies of any Rubric(s) to be used, and/or any additional supporting material(s)*** PROGRAM OVERVIEW Briefly summarize your assessment implementation and findings from the prior year, or prior years. How will past assessment activities and/or findings affect your assessment plans for the current academic year? If applicable, discuss any past curricular or instructional developments/changes that impact this assessment – e.g. you implemented changes based on assessment findings, and are now reassessing to determine impact of the changes; your previous assessment brought to light new/additional questions that will be addressed through the plan described below; your previous assessment indicated no changes were needed, so you are assessing a different outcome this year etc. This assessment plan is new and independent of prior assessments, and is focused on course to course knowledge retention in undergraduate majors in our department. NMSU Mission: New Mexico State University is the state’s land-grant university, serving the educational needs of New Mexico’s diverse population through comprehensive programs of education, research, extension education, and public service. Academic Program Mission: To improve our national recognition to match the quality of our program and to be a valuable resource to our state and community through service and outreach activities. To be one of the finest graduate and undergraduate teaching and research programs in the area of chemistry and biochemistry in the nation, and particularly in the southwest. Academic Program Assessment Report: Phase I Pg 1 of 8 10/31/2013 To be a leading place for faculty and students to come together and indulge in the passion of learning and discovery in a very supportive and stimulating atmosphere. Academic Program Goals: Our department’s academic program goals and objectives are currently under development. The following is a draft of our program goals: 1. To provide undergraduate majors (chemistry or biochemistry) a firm foundation in conceptual understanding and problem-solving skills related to both the fundamentals and applications of current scientific theories in their major (chemistry or biochemistry). 2. To assist majors in developing general problem solving, critical thinking, and analytical reasoning skills. 3. To train majors in the design and implementation of chemical/biochemical experiments, in the use of modern instrumentation and selected classical experimental techniques, and in experimental data collection, storage and analysis. 4. To develop students’ written and oral scientific communication skills. 5. To provide majors with experience and skills related to industrial or professional chemical or biochemical research. 6. To develop students’ information literacy skills relevant to chemistry and biochemistry. 7. To train majors in the proper procedures and regulations for safe handling, use, and disposal of chemicals. 8. To introduce majors to various ethical, historical, philosophical, and environmental issues related to chemistry and biochemistry. Academic Program Objectives: Formulation of 1. Majors will demonstrate proficiency in conceptual understanding and problem-solving skills related to both the fundamentals and applications of current scientific theories in their major (chemistry or biochemistry). 2. Majors will demonstrate proficiency in general problem solving, critical thinking, and analytical reasoning skills. 3. Majors will demonstrate proficiency in designing and implementing chemical or biochemical experiments, in the use of modern instrumentation and selected classical experimental techniques, and in experimental data collection, storage and analysis. 4. Majors will demonstrate proficiency in written and oral scientific communication skills. 5. Majors will demonstrate familiarity and basic skills with biochemical research. 6. Majors will demonstrate proficiency in information literacy skills relevant to chemistry and biochemistry through, for example, performing a literature search on a technical topic. 7. Majors will demonstrate knowledge and proficiency in the proper procedures and regulations for safe handling, use, and disposal of chemicals. 8. Majors will demonstrate familiarity with and be able to communicate clearly about various ethical, historical, philosophical, and environmental issues related to chemistry and biochemistry. Academic Program Assessment Report: Phase I Pg 2 of 8 10/31/2013 CURRENT YEAR – PLAN FOR ASSESSMENT Overview: We are initiating a multi-phase assessment of course to course retention of core concepts and skills among chemistry and biochemistry majors. In our department, faculty frequently observe that majors enrolled in required upper-division courses have significant deficiencies in prerequisite knowledge even though they have passed required prerequisite courses. Based on past measures of student performance in prerequisite courses, we believe that this is likely a knowledge retention issue. A chemistry and biochemistry course map is attached to this report as a supporting document. It illustrates the prerequisite connections and approximate sequence of study for all core chemistry and biochemistry courses. This assessment process will involve: 1. Measurement of direct student learning outcomes on core topics at two stages in the majors’ course sequence: i. Upon successful completion of “initial instruction courses” where the core topics are first taught. For the core topics addressed in our current-year plan, these courses are all part of our general chemistry sequence (CHEM 111-112-217 or CHEM 115-116). Measurements are planned in fall 2013 (CHEM 111, 112, 115, and 217) and spring 2014 (CHEM 111, 112, 116). ii. Upon entry into “applications courses” where knowledge and skills related to the core topics are necessary. For the core topics addressed in our current-year plan, there are several relevant courses (CHEM 356, CHEM 371, CHEM 313, and BCHE 395). Measurements are planned for spring 2014 (CHEM 313 and CHEM 371; BCHE 395) and fall 2014 (all courses) Once established, this assessment may be expanded to involve evaluation of student performance on core topics at two additional stages: iii. Upon exit from “applications courses”. This will give information on the degree of re-learning that happens in these courses. iv. Upon entry into “multiple exposure courses” for which applications courses are prerequisites. This will help assess whether multiple exposure to core topics improves course to course knowledge retention. 2. Cataloging of instruction times and practices for core topics in initial instruction courses for eventual correlation with knowledge retention data. 3. Development of a “test bank” of exam questions related to core topics. Direct Student Learning Outcome (1 is required and recommended): 1. Undergraduate chemistry and biochemistry majors should, upon completion of the general chemistry course sequence, be able to draw Lewis structures and determine molecular geometry and polarity for molecular substances based on their chemical formulas. Rationale: Molecular geometry and polarity are of foundational importance in theories of chemical reactivity and intermolecular interactions. Initial instruction courses: CHEM 111, CHEM 115 Applications courses: CHEM 313, CHEM 356 Academic Program Assessment Report: Phase I Pg 3 of 8 10/31/2013 2. Undergraduate chemistry and biochemistry majors should, upon completion of the general chemistry course sequence, be skilled in solving introductory-level problems related to acid-base equilibria. Rationale: Acid-base reactions have broad significance ranging from quantitative analysis and organic reaction catalysis in chemistry to protein conformation and enzymatic function in biochemical systems. Initial instruction courses: CHEM 112, CHEM 116, CHEM 217 Applications courses: CHEM 371, CHEM 395 What are the specific components you will be looking for in this outcome? Note: This is a fairly exhaustive list of specific components that may be narrowed based on data collection constraints. Core Topic #1: Drawing correct Lewis structures given a chemical formula. o Basic skill: structures with one or two central atoms for which the octet rule is satisfied o Advanced skill: structures with multiple central atoms or where the octet rule is not satisfied Determination of molecular geometry from a Lewis structure. o Basic skill: for structures with one or two central atoms and up to four electron groups per central atom o Advanced skill: for structures with multiple central atoms or 5-6 electron groups on a central atom Determination of molecular polarity from molecular geometry. o Basic skills: ranking of elements and bond dipole strengths by electronegativity, distinguishing polar and nonpolar molecules. o Advanced skills: ranking molecular polarities. Core Topic #2: Recognition of acids and bases from their formulas. Differentiation of strong and weak acid and base behavior. Determination of pH for solutions of weak acids and bases. Recognition of buffers based on chemical composition. Determination of pH for buffer solutions. Determination of acid or base concentration from titration equivalence points. Recognition of equivalence point(s) and buffer region(s) in titration curves. What evidence will be used to assess student learning on the identified outcome? (This could be a completed project, essay, presentation, solution to problem, etc. – something that students have produced.) Evidence of student learning in initial instruction courses will be obtained from and analysis of final exam performance. Evidence of student knowledge retention will be obtained from performance on entry exams or pretests in applications courses. Cataloging of instructional practices in initial instruction courses will be obtained by surveying course instructors. Academic Program Assessment Report: Phase I Pg 4 of 8 10/31/2013 Indirect Student Learning Outcome (Optional): none DATA COLLECTION: Which students will provide evidence? o Initial instruction courses: Data will be collected for all students completing the final exam in CHEM 111 and CHEM 115 (Core Topic #1) and for all students completing the final exam in CHEM 217 and CHEM 116 (Core Topic #2). Note: CHEM 116 is offered only in the spring semester, so data collection from this course will not occur in fall 2013. o Applications courses: Data will be collected for all students from entry exams (pre-tests) in CHEM 356 and CHEM 313 (Core Topic #1) and similarly in CHEM 371 and CHEM 395 (Core Topic #2). Note: CHEM 356 is offered only in the fall semester Who will collect the evidence? o o When? o o Data will be collected by course instructors. Preparation of entry exams for applications courses will be done in collaboration with Dr. David Smith to provide consistency with question types and difficulty in initial instruction course final exams. A database of questions ranked by difficulty (based on student performance) will be developed in support of this effort. Initial instruction courses: During final exam week, fall 2013 for all courses except CHEM 116. Data will be collected during the spring final exam week for this course. Applications courses: Planned for the first week of the spring, 2014 term for all courses except CHEM 356, where data will be collected during the first week of the fall, 2014 term. Specific scheduling is determined in collaboration with course instructors. Dates of completion of prerequisite courses will also be collected from students. How? From final exams (initial instruction courses) and entry exams (applications courses). How and by whom will the evidence be quantified? Evidence will be quantified by Dr. David Smith early in the Spring semester of 2014. DATA ANALYSIS: What are the defined levels of performance? (eg. Not evident, Inconsistent, Competent, Accomplished) Most or all individual questions will be graded as either right or wrong. Overall student performance levels: o Accomplished o Competent o Emerging competence o Deficient Academic Program Assessment Report: Phase I Pg 5 of 8 10/31/2013 What level of performance is considered evidence that a student has learned the intended outcome? (eg. “Competent” – see above) Based on a student’s average on all questions across a core topic: o o o o 85% or better: Accomplished 70% - 84%: Competent 50% - 69%: Emerging competence < 50%: Deficient Initial instruction course data will be used to measure initial learning competence. Applications course data will be used to measure knowledge retention competence. What number or percentage of students obtaining the desired level of performance is sufficient to determine the program is providing appropriate and effective learning experiences to achieve program expectations? (Benchmark) Benchmarks for knowledge retention competence will not be established until baseline performance has been measured. We have not quantified knowledge retention before, and really have no idea apart from anecdotal information ENGAGEMENT WITH FINDINGS/IMPACT: With whom will findings of the assessment be shared? (Who are your stakeholders?) With faculty of the Chemistry and Biochemistry department When? Within 1 month of submission of the next assessment report How? They will be presented at a faculty meeting. Recommendations will be developed in collaboration with the department’s undergraduate curriculum committee. How will you document engagement activities? This is still to be determined. How will the program/department use findings from the assessment to improve/support student learning? Correlation of knowledge retention findings with instructional practices may reveal issues related to knowledge retention. For example, does increasing total instruction time on a topic increase retention rates? This analysis will not be possible initially due to limited data on instructional practices prior to initiation of this assessment. Many factors have been found to correlate with knowledge retention. Investigation of these known factors will be used along with assessment findings to recommend changes in instructional practices to improve retention. For example, it might be recommended to include a class presentation on metacognitive skill development in general chemistry courses given that metacognitive skills are thought to enhance knowledge retention. Academic Program Assessment Report: Phase I Pg 6 of 8 10/31/2013 What opportunities for discussion and decision-making will be realized? “Closing the loop” in assessment – using findings to actually initiate change – is a daunting challenge. Faculty may feel threatened through assessment processes that reveal deficiencies. Focusing on knowledge retention across courses rather than learning in individual courses will, it is hoped, decrease anxiety levels and facilitate departmental teamwork toward program improvement. BEFORE SUBMITTING YOUR REPORT - Please attach copies of any/each rubric to be used, and any additional supporting material(s). Supporting Documents: 1. Chemistry and biochemistry course map. a. Blocks represent core CHEM and BCHE courses placed according to division and recommended year for completion assuming a four-year completion time. b. Lines connecting courses provide a map of all course prerequisites. c. “Initial instruction courses” are highlighted. Academic Program Assessment Report: Phase I Pg 7 of 8 10/31/2013 Academic Program Assessment Report: Phase I Pg 8 of 8 10/31/2013