Material Transfer Agreement (MTA) Request Form
advertisement

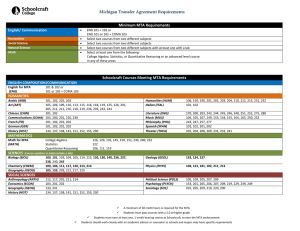
DELIVER PROVIDER’S MTA WITH THIS COMPLETED FORM TO: UIUC OFFICE OF SPONSORED PROGRAMS & RESEARCH ADMINISTRATION; gcoaward@uillinois.edu MATERIAL TRANSFER AGREEMENT (MTA) REQUEST FORM Principal Investigator: Phone: Email: Dept. Contact: Phone: Email: Provider: Contact Name: Phone: Project Title: Description of Material: E-mail: Deadline Date for Receipt of Material: Identify Banner FOAP to be charged for any shipping costs: Identify all funding sources and relevant grant codes or UIeRA # for the research involving this Material: Funding Source: Funding Source: Grant code or UIeRA #: Grant code or UIeRA #: YOU MUST OBTAIN APPROVAL BY THE APPROPRIATE CAMPUS REVIEW COMMITTEES PRIOR TO YOUR RECEIPT AND USE OF CERTAIN BIOLOGICAL OR HAZARDOUS MATERIALS. Do you have approval? ☐ Yes, Compliance Office: ☐ No, Reason ; Applicable protocol or registration # If the Material is regulated by law, check all that apply: ☐ Live vertebrate animals ☐ Human materials (including tissues, blood, blood products, bodily secretions or fluids, cell lines) ☐ Nonhuman primate materials (including tissues, blood, blood products, bodily secretions or fluids, cell lines) ☐ Human stem cells ☐ Animal stem cells ☐ Recombinant DNA Transgenic ☐ animals ☐ plants ☐ seeds Yes ☐ ☐ No ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ Biotoxins Pathogens ☐ human ☐ animal ☐ plant ☐ Biological hazard ☐ Pyrophoric gases ☐ Extremely toxic gases ☐ Explosives ☐ Dioxins ☐ Export controlled (ITAR/EAR) Do you intend to produce progeny or unmodified derivatives of the Material? Do you intend to modify, or produce modified derivatives of, the Material? If YES, briefly explain the modification: If YES, do you anticipate the modification having commercial value? Will the Material be used in conjunction with other materials from third parties? If YES, what are these other materials and who provided them? Was an MTA required? BE AWARE THAT YOU MAY NEED TO OBTAIN CONSENT FROM THIRD PARTIES BEFORE PROCEEDING. ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ Does your use of the Material relate to any previous or anticipated disclosures of intellectual property? Do you or any of the researchers have a financial relationship of any kind with the Provider? Will there be other UI researchers/graduate students in your lab using this Material? Do you intend to transfer this Material to a colleague? If yes, on campus; off campus Is the Material the subject of an FDA application, or is it regulated by the FDA? Is the Material commercially available? If the Material is highly proprietary, is there a substitute research tool that may be more readily obtained? Is there another source from which you could obtain the Material? ☐ ☐ If yes, identify the alternative source. PI Signature I certify that the information I have provided in this Request is true and accurate. My signature on the MTA will signify my agreement to direct the research in compliance with its terms and all relevant legal and ethical standards. I understand that OSPRA may need to negotiate the terms of the MTA with the provider. Signature: Date: ____________________________ OSPRA Form Ver. 140623