COMPANY: - ASQ Austin
advertisement

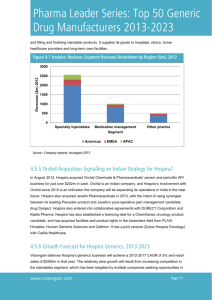
COMPANY: POSITION TITLE: Quality Assurance Site Compliance Director Hospira, Inc. REPORTS TO: Site Quality Director LOCATION: Austin, Texas SYNOPSIS: Hospira, Inc. seeks a Quality Assurance Site Compliance Director to provide internal and external oversight vigilance related to cGMP compliance to all activities required for products manufactured, tested, released, stored, and distributed by Hospira Austin. This position will lead the process toward regulatory compliance and will partner with manufacturing all cGMP initiatives. OUR CLIENT: Hospira, Inc. (NYSE: HSP) is a U.S.-based global pharmaceutical and medical device company headquartered in Lake Forest, Illinois. Worldwide sales in 2011 were approximately $4.1 billion. It has approximately 15,000 employees and is rapidly growing. Hospira, Inc. is the world's largest producer of generic injectable pharmaceuticals, manufacturing generic acute-care and oncology injectables, as well as integrated infusion therapy and medication management systems. Hospira's products are used by hospitals and alternate site providers, such as clinics, home healthcare providers and long-term care facilities. Hospira, Inc. helps hospitals help the hurting. The company, a 2003 spinoff of drug manufacturer Abbott Laboratories, develops, manufactures, and markets products that help improve the safety and productivity of patient care. Vision: Advancing Wellness™... through the right people and the right products The company's key values are based on the current strengths of its business, people and products, as well as the type of company Hospira, Inc. aspires to be. Hospira, Inc. will achieve its vision and deliver on its commitment through... Integrity -- Hospira, Inc. builds respect and trust in itself, its products and its employees by setting high standards and acting on its values. Ownership/Accountability -- Hospira's employees are its heart and soul. Its employees are counted on to advance the company's performance by meeting their commitments and keeping their promises. Speed -- Hospira's employees are empowered and expected to act quickly and decisively while making informed decisions and ethical judgments. Entrepreneurial Spirit -- Hospira, Inc. respects and encourages visionary thinking by embracing people who are passionate champions of creative ideas and who are willing to persevere on behalf of innovation. Injectables The company offers specialty injectable pharmaceuticals that include approximately 200 injectable generic drugs in multiple dosages and formulations; many of our products are available in popular differentiated delivery formats, several of which are proprietary, such as our ADD-Vantage® medication mixing system and our Carpuject® and iSecure™ prefilled syringes; proprietary specialty injectables, including Precedex, a proprietary drug for sedation; Biosimilars, which include Retacrit, a biosimilar erythropoietin, used primarily in the treatment of anemia in dialysis and in certain oncology applications; and Nivestim, a biosimilar filgrastim used for the treatment of low white blood cells in patients who have received a chemotherapeutic agent. It also provides intravenous solutions and nutritional products; and contract manufacturing services. In addition, Hospira, Inc. offers medication management products comprising infusion pumps and dedicated administration sets; Hospira, Inc. MedNet safety software system and related services; software applications and devices that support point-of-care medication administration; gravity administration sets; and other device products. Further, it engages in the development/co-development of proprietary pharmaceutical products, such as Precedex, a proprietary sedative; POSIDURTM, a long-acting version of the anesthetic bupivacaine; ATIR, a personalized hematology product designed for blood cancer patients in need of allogeneic bone marrow transplantation who cannot locate a matched donor; and Dyloject, a post-operative pain management drug currently awaiting FDA approval. I.V. Solutions, primarily a North American business for Hospira, Inc. , include large intravenous solutions and nutritionals — essential in virtually every aspect of hospital care. All of Hospira, Inc. 's injectable I.V. solutions include unit-of-use bar-code labels that can be used to support medication management efforts. Hospira, Inc. also offers infusion therapy solutions in its VisIV® nextgeneration non-PVC, non-DEHP I.V. container, an I.V. bag with advanced safety and environmentally friendly features. Device Medication Management Systems (MMS), the primary focus of Global Devices, plays a critical role in helping customers improve patient safety and enhance quality of care and clinician workflow. Our MMS portfolio includes a global installed base of approximately 550,000 infusion devices, including Symbiq®, our newest and most advanced general infusion device; the Plum A+® line of general infusion pumps; LifeCare PCA®, Hospira, Inc. 's pain management system; the GemStar® line of ambulatory infusion pumps; and other specialty devices. Integral to Hospira's MMS offering is Hospira, Inc. MedNet®, our drug-dose safety software that helps reduce medication errors by working to improve the intravenous medication administration process. In addition to tracking drug delivery data, Hospira, Inc. MedNet provides a useful tool for reporting and compliance purposes. Hospira's integrated MMS portfolio offers wireless, networking and several cross-platform interfacing capabilities to increase hospital utility, cost-effectiveness and interoperability with other hospital information technology systems. In addition, Hospira's expanding Client Services organization supports customers in maximizing the benefit of our systems. Clinical decision support and workflow tools offered by Hospira, Inc. include VeriScan® Rx, a handheld device that supports bar code and radio frequency identification (RFID) medication administration at the point of care and EndoTool® glucose management system, a clinical decision support software system that helps establish and maintain patient glycemic control in acute, critical care and operating room settings. Additionally, Hospira, Inc. recently acquired TheraDoc™ Infection Control Assistant™, providing continuous infection monitoring, intelligent alerts and timely analysis of hospital infections and TheraDoc Antibiotic Assistant™, providing real-time point-of-care screening to arm clinicians with timely and relevant information about antimicrobial resistance trends. In addition to MMS, Hospira, Inc. also offers gravity I.V. administration sets, critical care products and other device products, including LifeShield® products — a line designed to help hospitals take on risk-management issues and move toward better outcomes by controlling contamination and eliminating needlestick injuries and exposure to hazardous materials. Hospira's breadth of offerings help customers address the safety, productivity and cost of patient care. Used by hospitals worldwide, Hospira, Inc. products are also prevalent in outpatient clinics and other alternate healthcare sites. Its medication delivery systems include drug pumps, infusion therapy devices, and related medication management software. Its injectable drugs include cardiovascular, anesthesia, anti-infectives, oncology, analgesics, emergency and other areas. In addition, Hospira, Inc. provides contract manufacturing services for injectable pharmaceuticals. A good portion of Hospira's sales are to group purchasing organizations (GPOs), including Broadlane, Novation, and Premier. Austin, Texas Austin, the state capital of Texas, is on the edge of the "Texas Hill Country" and boasts rivers, lakes, scenic rolling hills, and a climate that encourages year-round outdoor activities. The city is home to the main campus of the University of Texas at Austin and offers an exciting array of restaurants, music, cultural events, and other recreational opportunities. It is billed as the “Live Music Capital of the World.” Austin also offers a low cost of living, no state income tax, affordable and spacious housing, a robust and expanding economy, and one of the best urban school districts in America. As a result, Austin is widely recognized as one of America's most livable cities and was recently listed in Money magazine’s feature article, “Best Places to Live” as the #2 best big city in America. School District http://www.austin.isd.tenet.edu/ POSITION SUMMARY: The Austin,TX injectable manufacturing site has almost 1500 employees and 160 in the quality organization, which is growing. The Austin facility provides: Extrusion and flexible IV bag fabrication; large volume liquid filling and terminal sterilization. Hospira is in the process of a global expansion and seeking the right people to drive the compliance activities at the plant. The Site Compliance Director is responsible for the development, management and continuous improvement of quality systems including compliance training, Corrective and Preventive Actions (CAPA), exceptions, investigations, quality and compliance metrics, audits, customer quality, etc. This Individual will oversee a divisional budget and manage a staff of 6-8 Direct Reports and a team of 15-20 Indirect Reports. The Site Compliance Director is responsible for raising Agency awareness by ensuring compliance in accordance with audits, FDA and Regulatory Visits and Global Regulators. All systems must comply with global regulatory requirements and result in product quality that exceeds customer expectations. This position will provide internal and external oversight vigilance related to cGMP compliance to all activities required for products manufactured, tested, released, stored, and distributed by Hospira Austin. This position will lead the process toward regulatory compliance and will partner with manufacturing all cGMP initiatives. ROLE EXPECTATIONS: Partner with functional teams to identify and implement optimal system designs Monitor new technologies to identify state of the art systems opportunities Maintain effective relationships with global regulators to understand current and anticipate proposed regulation to allow Hospira to remain in regulatory compliance Develop quality system talent to assure an ongoing supply of qualified candidates to meet business needs Develop, establish and maintain quality systems, policies, processes, procedures and controls ensuring that performance and quality of products conform to established standards and agency guidelines to ensure lasting customer satisfaction Oversee generation and review of documents used in good manufacturing practices; monitor audits of production and quality control areas Manage all regulatory and customer inspections for the site. Manage the compliance training function and ensure deployment of training systems globally. Interface with sites and functions to design and manage systems to elevate quality issues and drive corrective actions. Prepare and maintain the budget for all functions in the area LEADERSHIP COMPETENCIES: 1. Change Agent – This individual will work directly with the Plant Quality Assurance Director to help elevate the cultural quality and compliance standards of the facilities with respect to personnel and processes. 2. Team Building – This individual will model and “raise the bar” of Leadership through Team building and development. 3. Succession Planning – This individual will identify, cultivate and advance key leaders within the group for career succession. 4. Manufacturing Quality Process Improvement – This individual will integrate “best in class” thinking throughout the complete manufacturing process “above and beyond” cGMP standards. 5. Pro-Activity / Urgency – This individual will apply “critical thinking and analytical assessment” to his/ her management style to respond in a ‘constructive rather than reactive’ way to troubleshooting / problem solving. EXPERIENCE REQUIREMENTS: Requires a bachelor's degree in science or related field (Master's degree preferred) Minimum of 7-10 years of pharmaceutical and/or quality assurance experience Previous supervisory/management experience is required Current knowledge of cGMP's and regulatory changes in the industry are also required Manufacturing/packaging floor experience (IV injectables experience ideal) Must have excellent verbal and written communication skills Superior technical writing, problem solving and presentation skills are required. Individuals new to Futurestep must register at our web site: www. futurestep.com Interested and qualified candidates are welcome to submit a resume to: Lee T. King Consultant: Life Sciences & Healthcare Services Lee.King@futurestep.com Direct Dial: 203.406.8731 Cell: 914.629.2938 Peter S. Kaplan Sector Leader, Life Sciences & Healthcare Services Peter.Kaplan@futurestep.com Direct dial: 203-406-8769 Mobile: 914-329-3876