Cytoplasmic Inheritance: Extranuclear Genetics Explained
advertisement

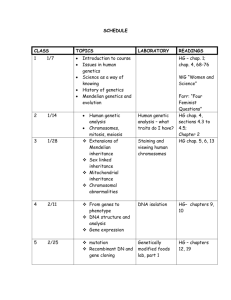
Cytoplasmic inheritance Cytoplasmic inheritance: Some self replicating genes (DNA) are present in the cytoplasm (mitochondrial DNA and chloroplast DNA) also. These are called plasmagenes and all the plasmagenes together constitute plasmon (like genome). The inheritance of characters by plasmagenes is called extranuclear or extrachromosomal inheritance. Eamples of examples of such inheritance common in nature (slide ppt), we ill critically explain 2 major groups the maternal inheritance (in snail) and the organelle inheritance. In the latter the DNA is present in mitochondria and chloroplast which controls the inheritance of some characters. A well known example of the characters controlled by chloroplasts is plastid inheritance in Mirabilis jalapa (4 O'clock plant). Other examples of organelle inheritance are i) iojap inheritance in maize,ii) inheritance of poky (imbalance in the mitochondrial physiology) in the fungus Neurospora crassa an iii) Petite in yeast, a mitochondrial character. iv) cytoplasmic male sterility in maize, is also a function of defective mitochondria. v) Several examples of such mitochondrial inheritance in human. Maternal inheritance. The amount of nuclear hereditary material contributed by the two sexes is almost equal but the cytoplasm in egg is always much more than that of the sperm. So in extranuclear inheritance, contribution of female parent is more. This is called maternal inheritance. The evidence of cytoplasmic or maternal inheritance is the coiling of shell in snails. When dextral Shell coiling in Limnaea peregra, a fresh water snail, is of two types, dextral ( = clockwise) and sinistral (anticlockwise). Dextral coiling is controlled by a dominant nuclear gene called D while sinistral coiling is due to a recessive gene. Since, zygote receives whole of its cytoplasm from the egg, the direction of shell coiling in the offspring is governed by cytoplasm of the mother. female (DD) is crossed with sinistral male (dd), all the offsprings of F1 generation (Dd) have dextral coiling. If sinistral female (dd) is crossed with dextral male (DD), the offspring have Dd genotype but coiling is sinistral. When the latter are self-bred, three types of genotypes are produced- 1 DD, 2Dd and 1 dd. However, shell coiling in all is dextral because the mother has Dd genotype. Result in F1 and F2 from a cross between dextral (DD) and sinistral (dd) snails Mitochondrial Inheritance Mitochondria are believed to have originated from an endosymbiotic event in early eukaryotic cell origins. A mitochondrion closely resembles an aerobic bacterium and the eukaryotic cell now relies on this organelle to be the primary producer of ATP which drives all cellular functions. The mitochondria has an outer membrane which resembles the other cytoplasmic membrane of the eukaryotic (host) cell and an inner membrane which more closely resembles the prokaryotic (bacterial) inner membrane where the oxidative phosphorylation enzyme complexes are found. Mitochondria, like prokaryotic cells, have double stranded "naked" circular DNA (mt DNA) which codes for its own 22 tRNAs, 2 rRNA's, and 13 proteins that form part of the oxidative phosphorylation complexes. These RNAs are transcribed and the proteins translated in the mitochondrial matrix. However, the nuclear DNA (nDNA) of the cell also codes for many of the proteins in the enzyme complexes. These nuclear gene products are translated in the cell cytoplasm on free ribosomes and have signal sequences which send them to the mitochondria. Mitochondrial disorders may be due to mutations in nDNA or in mt DNA. One can usually tell them apart by the pattern of inheritance. They both affect ATP production and, therefore, those tissues most dependent on ATP. Mutations in nDNA can interfere with the transport of mitochondrial proteins or iron into the mitochondria or between compartments, mtDNA replication, as well as the OXPHOS complex proteins themselves. Patterns of inheritance of mutations in nDNA that affect mitochondria include AD, AR or XL. Some of the mitochondrial disorders are expressed only in conjunction with a nuclear gene mutation. The two locus model applies to LHON which is expressed only if the person inherits a mutant XL gene and sensorineural deafness which is expressed only in the presence of two doses of an AR expressed gene. Interestingly, both of these traits are associated with homoplasmy (all of the mitochondria in all cells are mutant or homogeneous) whereas most mitochondrial disorders show heteroplasmy (there are varying proportions of mutant and normal mitochondria in the cells of the individual). Mitochondrial inheritance is exclusively through the mother. This is because all of the mitochondria in the zygote come from the egg cytoplasm. The sperm contributes only its nucleus. (If an occasional paternal mitochondria were to be included it would be diluted out by the large numbers of mitochondria present in the egg.) Pedigrees will show both male and female affected but only the females pass the disorder on. The pedigree can look superficially like an XR pedigree but there is no male transmission through carrier daughters. The human primary oocyte has about 100,000 mitochondria but loses most of them during maturation. The result is a stage in which there are between 10 and 100 mitochondria. During cleavage of the zygote, the number is built up to about 10,000 mitochondria per cell. The severe reduction of mitochondria in the oocyte is referred to as a "bottleneck". If a mutant mitochondrion is included in the 10 to 100 in the oocyte there is a chance that it will be over represented in the cells of the embryo. Mitochondria are randomly distributed into daughter cells, thus by chance alone some cells may receive more or less of the mutant mitochondria. Those tissues that receive a larger amount of defective mitochondria will have a lower ATP production. If the tissue is one which requires a large amount of energy such as cell of the nervous system, muscles, kidneys, etc., the person with the mutant mitochondria will be affected. The term heteroplasmy is used to describe the situation where a cell or tissue contains both mutant and wild-type mitochondria. The term homoplasmy describes the situation whereby all the mitochondria have the same genome, either wild-type or mutant. Sporadic cases of mitochondrial diseases are due to a new mitochondrial mutation in maternal mitochondria. The new mutation may be present in a minority of her cells but if they are in some of her primary oocytes she can pass them on. Therefore, an (apparently) unaffected mother can pass on mutant mitochondria in higher numbers than were present in her since the formation of an egg represents a "bottle neck" where the mutant mitochondria can be disproportionally included in the egg as compared with her other cells. Then these mutant mitochondria can multiply to produce a greater proportion of the 1000's of mitochondria in the sporadic cases known. A two locus model has been proposed to explain the inheritance of LHON-mitochondrial inheritance coupled with an X-linked gene explains the lack of affected offspring of some affected females, the slight excess of males, the later onset in females, and the pattern of affected females in pedigrees is accounted for by unfortunate X chromosome inactivation in females who are heterozygous for the X-linked gene. Affected people are homoplasmic for the mt mutation most of which are due to one of 2 or 3 point mutations in respiratory chain proteins. There is more than one mt mutation that can cause LHON (allelic heterogeneity); however, in any one family all affected family members will have the same mutation. For the most part LHON is a tissue specific mitochondrial disorder of the eye although cardiac dysrhythmia is frequently associated with the optic neuropathy but there is no skeletal muscle involvement. Less variation in this mt disease; some variation in age of onset and severity. Sensorineural deafness. One form of sensorineural deafness is due to homoplasmic mt mutation coupled with an AR gene. Pearson marrow-pancreas syndrome. Pearson Marrow-pancreas syndrome is a severe anemia in infancy with exocrine pancreatic dysfunction. Mt DNA deletions have been demonstrated. Mutations in nuclear genes coding for oxidative-phosphorylation subunits also cause mitochondrial disorders. Leighs disease (AR) is an invariably fatal degenerative brain disease. It is caused by a deficiency in a Complex IV subunit (cytochrome oxidase). The patients have encephalopathy with seizures, ocular and respiratory problems. There is an interesting AR disorder called the Mitochondrial Depletion Disease. The defect is due to a defect in the mt DNA replication whose replication enzymes are all coded for by nuclear genes. One finds mitochondria with no mt DNA. In one family 2 sisters died of the mitochondrial myopathy and a cousin from liver dysfunction. The defect is in the regulation of the mt DNA copy number. Environmental factors can mimic these disorders (phenocopies). Drugs that interfere with mt DNA replication such as AZT which is a base analog can cause myopathy, RRF and weakness. Some antibiotics are known to cause deafness by damaging mitochondrial function. Some interesting questions are: Why do only dup/del mutation and not base substitution mutation in mt tRNA cause CPEO? Why is only the optic nerve and sometimes the heart affected in LHON if all cells are homoplasmic? Why do you find homoplasmy only if nuclear genes are involved? Attempts at therapy (RX) try to boost ATP production by giving electron shuttling substances such as coenzyme Q (which also stabilizes the inner mt membrane), succinate (substrate), riboflavin (FAD, coenzyme), Vitamin C and Vitamin K. The last four all act as surrogate etransport molecules in specific area of the electron transport system (ETS). Veterinarians are aware of a mitochondrial disorder in Holstein cows. It is a heteroplasmic mt DNA point mutation with considerable variation between sibs. New areas of interest that appear to be associated with mitochondrial defects are Alzheimer, Parkinson and aging. Mt DNA has also played an important part in exploring human evolution. The "mitochondrial Eve" is believed to have originated in Africa. Mt DNA has the advantage of being haploid and only inherited from one parent (the Y chromosome has also proven useful for the same reason). The timing of evolutionary divergence is done by following the number of mutations that have accumulated, a DNA clock. The technique has proven useful in looking at the evolutionary divergence of other closely related species such as dogs and wolves. Comparison of the sequence of mt DNA from the skeleton believed to Czar Nicholas, the exhumed bones of his younger brother, and the mt DNA from two living relatives showed a rare mt DNA mutation present heteroplasmically in the two brothers' skeletons and homoplasmically in the two living relatives. There are many more examples of human evolution studies and forensic uses of mt DNA. Mitochondrial DNA Diseases This table lists only some of the disorders that can be caused by mutations in mitochondrial DNA. Certain of these conditions can also be caused by nuclear mutations or other processes that hinder mitochondrial function. Mitochondrial Disorders and Disorder Features Alzheimer's disease (in some cases) Progressive loss of cognitive capacity CPEO (chronic progressive external ophthalmoplegia) Paralysis of eye muscles and mitochondrial myopathy Diabetes mellitus (in some cases) High blood glucose levels, leading to various complications Dystonia (some cases) Abnormal movements involving muscular rigidity; frequently accompanied by degeneration of the basal ganglia of the brain KSS (Kearns-Sayre syndrome) CEO combined with such disorders as retinal deterioration, heart disease, hearing loss, diabetes and kidney failure Leigh's syndrome Progressive loss of motor and verbal skills and degeneration of the basal ganglia; a potentially lethal childhood disease LHON (Leber's hereditary optic neuropathy) Permanent or temporary blindness stemming from damage to the optic nerve MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke like episodes) Dysfunction of brain tissue (often causing seizures, transient regional paralysis and dementia) combined with mitochondrial myopathy and a toxic buildup of acid lactic acidosis and in the blood MERRF (myoclonic epilepsy and ragged red fibers) Seizures combined with mitochondrial myopathy, may involve hearing loss and dementia Mitochondrial Myopathy Deterioration of muscle, manifested by weakness and intolerance for exercise; muscle often displays ragged red fibers, which are filled with abnormal mitochondria that turn red when exposed to a particular stain NARP (neurogenic muscle weakness and retinitis pigmentosa) Loss of muscle strength and coordination, accompanied by regional brain degeneration, ataxia and deterioration of the retina Pearson's syndrome Childhood bone marrow dysfunction (leading to loss of blood cells) and pancreatic failure; those who survive often progress to KSS SCIENTIFIC AMERICAN August 1997 ADDENDA As I mentioned in my Addenda in the Chromosome Abnormalities lecture (#5)) I am often contacted by parents of with children with genetic disorders as a consequence of this web site.The following is my response to a mother with whom I had been in contact regarding her daughter who clinically appears to have Angelman syndrome. We saw her daughter in our pediatric genetic clinic after which we ran some standard tests for Angelman all of which came out negative My Response to a mother of an Angelman child: Angelman results when a child does not receive a normal UBE gene from the mother. The father's UBE gene is normally turned off (silenced) during spermatogenesis....when the sperm is formed. In normal individuals they have one normal UBE gene from the father and one deleted or silenced gene from the mother. The (normal) silencing of certain genes from either the mother or father is referred to as genomic imprinting and it occurs during egg and sperm maturation prior to conception. Angelman syndrome is due to the abnormal silencing of a gene that needs to be turned on in order to be totally normal. As mentioned above, the gene involved is ordinarily "turned off" in the male by a process known as methylation. (Methylation is a process the cells use to silence genes.) If a child does not get the normal "turned on" gene from the mother, the child has Angelman syndrome. The loss of this maternal gene activity can be due to several different errors. First of all there can be a deletion of the maternal gene...even just part of it being deleted is enough to cause the problem...so if the child of the woman who wrote the letter is "deletion positive" it means her child is missing the necessary gene and thus has Angelman syndrome. It appears from her letter that she does not understand what "deletion positive" means and is looking for a "cure" that would not be available to a child with a deletion. Angelman can also arise due to the abnormal methylation of the normal maternal gene (resulting in the silencing of the normal gene). The first test I had done on your child would have detected either a deletion and an abnormal methylation pattern but it did not. Another possibility is that a child has inherited two chromosomes 15 from the father instead of one from the mother and one from the father. This is called "uniparental disomy". In these cases the child has two methylated (silenced) genes and no normal one from the mother. Another possibility is that there is an "ordinary" mutation in the UBE gene that does not allow it to make its normal product. The second test I ordered for your child was a DNA sequencing of the gene in an attempt to see if there a mutation but no mutation was detected (however, some mutations can be missed by this test). The next tests I proposed to you included looking for the deletion in SOME of her cells. This is called mosaicism. It could be that the gene is missing from some but not all of her cells. This situation would have occurred after conception. Another test I proposed for your child was to look at the Rett gene (MEPC) since Rett syndrome overlaps with Angelman in the clinical symptoms. Rett syndrome is caused by a defect in one of the many genes involved in the normal methylation/silencing of many genes. The clinical picture is severe which gives you an idea of how important the normal process of methylation is during development. The Rett gene is on the X chromosome and if a male fetus gets the mutated gene it dies in utero. Females who have the mutation on one of their two X chromosomes will survive (but be seriously affected) since the "good" gene on the other X chromosome can compensate for the "bad" one to some extent. While individuals with defects in the same gene are expected to overlap in their symptoms, there is, of course, variation in the expression of all genetic disorders. This is because there are many more genes in the individuals and of those thousands of other genes some can modify the deleterious effects of the mutation. Even Down syndrome children who all have an extra chromosome 21 and have similar appearance and problems vary in their abilities due to their other genetic makeup and due to the care they receive as they are developing. Another source of variation is that In single gene defects there are many different mutations possible in the single gene involved and thus variation in expression (genes are very large). Also there is much overlap among disorders so mutations in totally different genes can produce similar or overlapping clinical symptoms (like Rett and Angelman). It is easy for the layperson to get confused and I am certain I could explain this to you better on a one to one basis. It's a bit difficult by e-mail. I think the person who wrote the letter you refer to is trying to make connections from different sources of information and is getting a little confused herself and that is why you cannot understand her. These are complex issues and complex papers which are difficult for non-geneticists to decipher. I printed a copy of the paper to which she refers and I can try to explain it to you later. The paper is talking about an overlap in symptoms with disorders that involve "imprinting" errors. Because the MECP2 gene is involved in methylation in general any mutation in that gene would be expected to have some effect on all genes that are methylated. The UBE gene is only one of them. The letter writer mistakenly believes that the silencing of the paternal UBE gene occurs only in the brain. Actually, the paternal UBE gene is silenced in every cell in the individual's body. Being able to turn on the paternal gene in a person with Angelman would be a solution but no one has found a way to do that yet. I doubt your obstetrician would have read the article I mentioned to you on ART (assisted reproductive technology) but here is the reference: Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004 Apr;74(4):599-609. The article is talking about IVF (in vitro fertilization) and ICSI (intra cytoplasmic sperm injection). However since their main point is that neither of those techniques allow normal processing of the egg or sperm at a time when genomic imprinting (demethylation and remethylation) is normally occurring there is a greater than expected incidence of epigenetic errors and disorders associated with them such as Angelman, Beckwith-Wiedeman, and retinoblastoma. I suspect that inducing ovulation (using clomid or other similar medications) which speeds up the egg maturation and release would also interfere with the normal reprogramming of methylation patterns that occurs during normal gametogenesis. Clomid is not mentioned specifically in the article but it follows from their reasoning that any interference with normal egg (or sperm) maturation could cause imprinting problems. Topics: Genetic sexing, Meiotic drive, Conditional lethal, Cytplasmic incompatibility, SD system, PM dysgenesis.