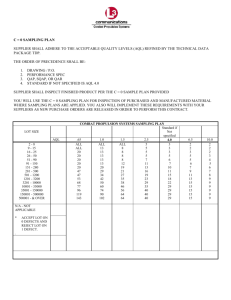
IMOS National Reference Station Field Sampling
advertisement

INTEGRATED MARINE OBSERVING SYSTEM NATIONAL REFERENCE STATIONS BIOGEOCHEMICAL OPERATIONS A PRACTICAL HANDBOOK CSIRO Marine and Atmospheric Research Laboratories Report for Editor G. W. Critchley Contributors: CSIRO Marine and Atmospheric Research: Abell, G., Allen, S., Berry, K., Bonham, P., Clementson, L., Coman, F., Critchley, G., Frampton, D., Latham, V., Richardson, A., Robert, S., Terhell, D., Thompson, P.A., Tilbrook, B. and Sherrington, R. Flinders University & SARDI: Seuront, L. N.S.W. Department of Environment and Climate Change: Pritchard, T., Ingleton, T. Australian Institute of Marine Science: Bainbridge, S., Skuza, M., Steinberg, C. University of Tasmania – eMII project: Magierowski, R., Roberts, K., Tattersall, K. Version 2.2 (July, 2012) NRS Sampling Manual Page 1 National Reference Stations INTEGRATED MARINE OBSERVING SYSTEM IMOS BIOGEOCHEMICAL OPERATIONS MANUAL Introduction: An often understated part of experimental and observational protocols is that part played by sampling correctly. If proper procedures for sampling are not followed then even the most meticulous of laboratory analysts and finely tuned apparatus are all worthless and have little meaning – it can quite rightfully be said that: “no data is better than bad data”. For this reason, it is recognised that IMOS Biogeochemical samplers’ annual training workshops are conducted; with the sampling team members rotating through on a regular basis to ensure consistency in sampling technique is maintained nationwide and regular networking of the participants can occur. The following procedures and methodologies for biogeochemical sampling, sampling regimes, analyses and data flow conducted to meet the requirements of the Australian IMOS NRS project are laid out purposely in detail. The steps in the body of this document are to be followed to ensure that the best and most consistent quality samplings between sites are acquired, thus leading to quality data being obtained from the ensuing analyses. This manual has come about from an initial scoping meeting where it was decided that not all parameters that were desired could effectively and logistically be obtained. Those that were considered the most important and achievable are those that are enclosed herein. A person following the proper and agreed procedures – with a particular emphasis on sampling techniques – as outlined in the body of this handbook will give the laboratory analysts the best opportunity to obtain quality data. In some instances there are deliberate repetitions of some directions, which have been included to assist in bringing attention to particular details that should be observed – and why. The sampling and analytical regime that is described in this document utilises the same “blue water” methods for collection and analysis as on larger vessels such as the” Australian Marine National Facility – Southern Surveyor”. However owing to the smaller size of the vessels involved in the Australian IMOS ANMN NRS Biogeochemical sampling, there are limitations as to what can and cannot be achieved – e.g. the use of niskin style sampling bottles on a wire cable, as opposed to the use on larger vessels of a “real time” rosette sampler. Of the nine NRS, three build on Oceanographic time series data collection sites that go as far back as the early 1940’s. The concept of the IMOS NRS Biogeochemical sampling is to enhance the existing and expand the national coverage of time series data. The manner in which this manual has been laid out enables it to be “broken up” into smaller pieces, for example: “detailed sampling procedures”, or “pre-analyses sample treatment”, etc. without the necessary need of a large document. Every endeavour to achieve monthly collection of biogeochemical data from each site, based within the major continental boundary currents, will be made in order to obtain monthly, seasonal, annual and long term variability or shifts in the Australian marine ecosystem – in particular as a response to Climate Change. NRS Sampling Manual Page 2 In addition to the monthly sampling there will be co-located moorings with instrument arrays at 2 depths – some with a third surface meteorological surface buoy – that are deployed at the National Reference Stations; recording a suite of time series data which will be complementary to the discrete monthly water column samples. The moored instruments are Sea-BirdTM sensor packs modified by WetLabsTM, and as a package they are marketed as Water Quality Meters (WQM’s). These packages measure: Conductivity, Temperature, Depth, Time (UTC & Local), Dissolved Oxygen, Fluorescence and Turbidity at two set depths only. The biogeochemical results will be used to monitor and assess the performance of the moored WQM’s as well as creating a large independent suite of other extremely valuable data obtained from a number of depths that cannot be sensor determined. From the outset, this manual has been written as a hands on guide to the acquisition of quality samples and hence quality data. It is not a “research” publication as such; it is more a collation of the best techniques in practice in today’s Biogeochemical and bluewater Oceanographic community for ensuring the output of reliable, quality data to the end-user community. The aim is for the sampling, analytical, and reporting standards to be at least equivalent to: the WOCE (World Ocean Circulation Experiment) and JGOFS (Joint Global Ocean Flux Study) studies. IMOS is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy and the Super Science Initiative. www.imos.org.au The National Reference Station information may be found at the following link: http://imos.org.au/anmnnrs.htmL NRS Sampling Manual Page 3 As stated previously, the NRS sites will be sampled monthly – weather and remoteness of locality permitting – for biogeochemical data; with mooring servicing intervals yet to be fully resolved. It is not within the scope of this manual to cover the use of, acquisition, downloading, storing, calibrating or doing QA/QC on data acquired from the profiling CTD’s in use on the NRS vessels or the NRS WQM moorings. Another short companion manual is planned to be produced in the near future, to accompany this one: “Profiling CTD Guidelines for IMOS National Reference Stations”; which will deal with the basic operation of profiling CTD’s at the NRS sites and their set up for NRS requirements. There must be standardised site coding for site identification and field sheets and sampling labels used consistently and correctly. Sample collection methodologies for the suite of biological and physical parameters; including sample preservation must be followed consistently at all sites. Good collection techniques will ensure good samples for analyses . Sample storage and transportation to points of analyses also need to be strictly adhered to Analytical protocols for the above biological and chemical parameters must be consistent and if carried out at more than one laboratory – would require many inter-laboratory comparisons to be run; hence this spread of analytical laboratories has been kept to a minimum and these already participate in international comparison trials. Standardised units of measurement appropriate to each biological, chemical and physical characteristic have been agreed to and applied. Accepted SI units should be used wherever possible. It is also necessary to set standardised processing procedures in place for each water and biological parameter which includes quality assurance and quality control. Each data point/set that has been sampled, analysed, processed and checked for QA/QC at all stages of collection and analysis is required to be entered into a standard national database (eMII), with fully descriptive metadata; which may include a short report if necessary, for each data set which will be openly accessible to all participants and the end users of the collected data – which includes free and open access to any party who may wish to access and utilise the data. I would like to express my personal thanks to Mr. S. Allen, Technical Director, IMOS, for the faith shown in me for the methods description and successful implementation of a functional Biogeochemical sampling and data gathering project for IMOS I would like to express my personal thanks to Dr. T. McDougall for his support. NRS Sampling Manual Page 4 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Figure 1, below, shows the location, and geographical spread of the 9 NRS Biogeochemical sampling stations, accurately charted using decimal degrees. Refer to Table 1, following Fig.1, for the actual latitudes and longitudes of each site. There is currently consideration being given to introduce another 2 or 3 National Reference Stations, however dependant upon their remoteness, they may not be able to be fully sampled for Biogeochemical data on a monthly basis and the sampling may occur only at changeover times of double depth WQM moorings. Fig. 1 NRS Sampling Manual Page 5 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL The biogeochemical sampling stations which are complemented by the NRS moored network are shown below in Table 1. The table is shown in the operations manual for the interest of all those involved with the sampling and shows the geographic spread of the National Reference Stations. It was intended that the 9 station roll-out be covered over 2 financial years, with 6 being the target for commencement in the first fiscal year 2008/2009. However, with a lot of effort and overcoming the many logistics of sampling and sample movement to overcome, it was possible to exceed this target and fully kit out 8 (in the first fiscal year of the proposed 9 over 2 fiscal years) National Reference Stations for Biogeochemical operations. Table 1. The National Reference Stations codes, depths, locations (Decimal Degrees) Site Node Station code State Start-up Date Nominal Sonic Depth Nominal Longitude Nominal Latitude Maria Island* CMAR MAI TAS Oct 1944 90m 148.233333 -42.596667 Kangaroo Island SARDI KAI SA New 100m 136.448 -35.836 Esperance CMAR ESP WA New 50m 121.85 -33.933333 Rottnest Island* CMAR ROT WA Apr 1951 50m 115.416667 -32 Ningaloo CMAR NIN WA New 50m 113.94665 -21.871733 Darwin AIMS DAR NT New 20m 130.7827 -12.417467 Yongala AIMS YON QLD New 28.9 147.26 -19.306 North Stradbroke Island CMAR NSI QLD New 60m 153.580217 -27.388917 Port Hacking 100* DECC PHB NSW May 1953 100m 151.25 -34.083333 With WQMs *Denotes long term stations already sampled and analysed historically by CMAR – some dating back to the 1940’s. NRS Sampling Manual Page 6 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Standard Sampling Depths for water chemistries, other discrete depth sampling and phytoplankton (except Carbon – see table 3) The following table (Table 2.) shows the sampling depths for the water chemistry and phytoplankton sampling, with the excess (residual) left in each of the niskins (sampled to 50m), measured, recorded and placed into a 20l carboy – based on the surface niskin residual volume – to give an “integrated water column” sample. This is often the only cast for a station, unless they are in deeper locations. These previously determined depths, laid out in Table 2 are carried out to give coverage of the photic zone – of course some of the stations are sampled deeper than shown here (see table 4). Because the only Carbon sampling depth common to all sites is the surface niskin sampling (See Table 3); It is necessary to record the residual volume to be transferred to the composite sample carboy from each subsequent niskin, based on the residual left after sampling from the surface bottle. By basing the collection of the residual volumes for the other niskins upon the surface bottle, it avoids biasing the “integrated/composite” carboy sample - as there will be greater residuals in niskins not sampled for Carbon. Table 3 lists all the Carbon sampling depths – which do put constraints on the total water budgets for the 5l niskins. Any water left in a niskin after the niskin sample volume is measured as equivalent to the surface residual and added to the carboy, can then be discarded. Table 2 Site Station code Sonic Depth Officer Responsible Biogeochemistry sampling depths (excluding carbon) – First Cast Maria Island* MAI 90m Tim Lynch Surface, 10, 20 , 30, 40, 50 Kangaroo Island KAI 110m Charles James Surface, 10, 20 , 30, 40, 50 Esperance ESP 50m Tim Lynch Surface, 10, 20, 30, 40, 50 Rottnest Island* ROT 50m Tim Lynch Surface, 10, 20, 30, 40, 50 Ningaloo NIN 50m Tim Lynch Surface, 10, 20, 30, 40, 50 Darwin DAR 25m Craig Steinberg Surface, 10, 20 , bottom + 2.5 (2) * Yongala YON 28.9 Craig Steinberg Surface, 10,20 , bottom + 2.5 (2) * North Stradbroke Island NSI 60m Anthony Richardson Surface, 10, 20, 30, 40, 50, 60 *** (Single bottles – now WQM’s yet) Port Hacking 100* PHB 100m Tim Pritchard Surface, 10, 20 , 25, 30, 40, 50 ** With WQMs NRS Sampling Manual Page 7 Notes regarding Table 2, above * NOTE: DAR & YON are shallow sites which will have to do a (measured) double collection to ensure there is enough sample water in the ”water column” carboy for later sample preparation for later analyses. ** NOTE: PHB, 25meter sample water is not to be measured nor added to the water column carboy, but still sampled for other parameters for historical reasons *** NOTE: NSI – does not include 60m water in the water column carboy • Note: Due to the historical nature of some of these stations they cannot be sampled with niskins at 15m and 20m depths for example. If an historical station has been consistently sampled at 20m, or in the case of PHB (25m) the historical depth must still be sampled at that depth for retention of data continuity. At the next cast, collect the other water chemistry samples deeper than 50m where they are required or possible – for a particular site. For each site the sampling vessel’s capability will determine whether it is possible to gather all the depths for the biogeochemical suite, to the bottom in one cast, and then conduct a further cast for the two WQM (5l) samples. It may turn out that a third cast is necessary for deeper stations if the vessel is of limited capability for the particular NRS it will be used for. NRS Sampling Manual Page 8 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Table 3 – Sampling Depths for CARBON SAMPLES which are taken, in addition to the standard chemical parameters (Table2) from the niskin bottles Note: Samples are collected from the near-bottom Niskin bottle and the Niskin bottle above it, in order to determine if there are intrusions of CO2 rich water onto the shelf. If sampling depths are shallower, according to the sonic depth, than as shown in the table, please ensure the two deepest samples are collected Table 3 Site Station code Sonic Depth Officer Responsible Biogeochemistry sampling depths (excluding carbon) – First Cast Number/total Carbon sampling depths per site Maria Island* MAI 90m Tim Lynch Surface, 10, , 30, , 50, 75, bottom + 2.5 6 Kangaroo Island KAI 110m Charles James Surface, 10, , 30, , 50, 75, 90, bottom + 2.5 7 Esperance ESP 50m Tim Lynch Surface, , 20, , 40, bottom + 2.5 4 Rottnest Island* ROT 50m Tim Lynch Surface, 10, , 30, , 50, bottom + 2.5 5 Ningaloo NIN 50m Tim Lynch Surface, 10, , 30, , 50, bottom + 2.5 5 Darwin DAR 25m Craig Steinberg Surface, 10, 20, bottom + 2.5 4 Yongala YON 28.9 Craig Steinberg Surface, 10, 20 , bottom + 2.5 4 North Stradbroke Island NSI 60m Anthony Richardson Surface, 10, , 30, , 50, bottom + 2.5 5 Port Hacking 100* PHB 100m Tim Pritchard Surface, , 20 , , 40, , 60, 80, 100, bottom + 2.5 7 With WQMs Due to variation of depths at different sites requested by the Carbon sampling group, as shown in the above Table 3, there will be unique station field logs and labels created that are tailored for use at each unique site. They will clearly identify which sample depth is sampled for each parameter type and cover all necessary unique information for any particular sampling at any particular site. NRS Sampling Manual Page 9 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Table 4 – indicates the sampling depths for water chemistry and Carbon samples (See Table 3) from deeper than ~ 50m, which may not be obtained concurrently from the first niskin cast to capture the < 50m “integrated water column” carboy water. As mentioned earlier, dependant upon the sampling vessel capabilities, these may be conducted on separate casts. It is also not necessary to keep any of the residual niskin waters after sampling these depths (> 50m.) Note: Samples are collected from the near-bottom Niskin bottle and the Niskin bottle above it to determine if there are intrusions of CO2 rich water onto the shelf. Again, if the sonic depths are shallower than indicated in the table, please ensure the two deepest samples are collected Table 4 Site Station code Sonic Depth Officer Responsible Casts for chemistries >50m Maria Island* MAI 90m Tim Lynch 75, 100, bottom + 2.5 Kangaroo Island KAI 110m Charles James 75, 90, bottom + 2.5 Esperance ESP 50m Tim Lynch N/A Rottnest Island* ROT 50m Tim Lynch N/A Ningaloo NIN 50m Tim Lynch N/A Darwin DAR 25m Craig Steinberg N/A Yongala YON 28.9 Craig Steinberg N/A North Stradbroke Island NSI 60m Anthony Richardson (60m already sampled as in Table 2) Port Hacking 100* PHB 100m Tim Pritchard 60, 80, 100, bottom + 2.5 Sampling depths per site (metres) With WQMs NRS Sampling Manual Page 10 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Table 5 – explains the requirements for WQM biosensor calibration. As 20m will also likely correspond to the surface WQM (at most stations), calibration/sensor samples are required, and so a further cast must be conducted in order to capture some extra samples for WQM performance monitoring. It is not necessary to repeat any of the other shallower chemistry samplings. From the depths of the upper WQM and the deeper WQM (at bottom +2.5m), sampling at the particular mooring site of 5litres is required for HPLC pigment analyses for WQM calibration. Table 5 Site Station code Sonic Depth Officer Responsible Casts for WQM pigment comparisons and Genomics samples Sampling depths per site (metres) With WQMs Maria Island* MAI 90m Tim Lynch 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Kangaroo Island KAI 110m Charles James 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Esperance ESP 50m Tim Lynch 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Rottnest Island* ROT 50m Tim Lynch 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Ningaloo NIN 50m Tim Lynch 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Darwin DAR 25m Craig Steinberg = surface WQM (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics Yongala YON 28.9 Craig Steinberg = surface WQM (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics North Stradbroke Island NSI 60m Anthony Richardson A moored WQM system will be installed in 2009/10, so: 5 litres surface for genomics, until mooring deployment when it will be required to take 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only) Port Hacking 100* PHB 100m Tim Pritchard 20 (WQM Calibration only), bottom + 2.5 (WQM Calibration only), 5 litres surface for genomics NRS Sampling Manual Page 11 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL OUTLINE of Sampling Regime Parameters to be sampled are: 1. Carbon Parameters: • Total Dissolved Inorganic Carbon (TCO2) • Total Alkalinity (TALK) 2. Hydrochemical parameters: • • • • • • • Dissolved Oxygen (chemical) – only conducted at 2 stations salinity Nitrate/nitrite silicate phosphate ammonia total, organic and inorganic suspended matter 3. Biological parameters: • Phytoplankton • pigment composition • phytoplankton microscopy (species composition) • flow cytometry • Zooplankton • dry weights • community composition • average size of the zooplankton community 4. Physical/profiling instrument measurements • Secchi disk – black and white • CTD with profiling capabilities for: • temperature • pressure (depths) • conductivity • fluorescence • turbidity • Dissolved Oxygen (to be retrofitted) 5. Sampling for Genomic analysis • Zooplankton • Microbial (including phytoplankton) NRS Sampling Manual Page 12 IMOS National Reference Station Field Sampling A Suggested Field Sampling Order • Using GPS, locate the site of the Reference Station. • Ensure the log sheets are filled completely, correctly and with all possible information – including Times and Dates in UTC – upon arrival, and as sampling proceeds. This is very important as the metadata is sourced, by data harvesting software, from these records. • Secchi disk • Conduct a CTD cast (with ancillary sensors) to obtain a water column profile to 2.5m from the bottom – to coincide with the bottom moored WQM (WetLabsTM Water Quality Meters). • Check if the CTD cast was successful, by connecting the CTD to the laptop and check to see if data were successfully obtained. If not, repeat the profile. • Take 3 zooplankton drop net samples for: • • • • Zooplankton biomass (this is a destructive analysis) – preserve with formalin in an opaque plastic container and sealed with tape Species composition (microscopic analysis) – preserve with formalin in an opaque plastic container and sealed with tape Zooplankton genomics sample is stored in a black jar, kept cool and is later prepared during the on shore sample processing for storage in liquid nitrogen Water chemistries drawn directly from the racked Niskin bottles: • Usually the first wire cast using 5 litre Niskin bottles will be taken at (just below the) surface, 10m, 20m, 30m, 40m, 50m, (or as otherwise determined by site characteristics, vessel capability and a stations historical record). • After the niskin cast has been triggered by messengers and the bottles retrieved and placed in a bottle rack; from each Niskin bottle take discrete samples in the following order for: • chemically determined Dissolved Oxygen – which will be sampled only at 2 stations, where there is minimal sample transport involved • CO2 (TCO2, and Total Alkalinity) requires ~1500mL (including rinses) from niskins at nominated depths (refer to Table 2 and 3; and each site specific log sheets) • Other water chemistries – salinity, triplicate nutrients, use ~1000 mL. from each niskin (see Table 2 and 4) NRS Sampling Manual Page 13 • • Phytoplankton, pigments, picoplankton, Suspended solids – “Integrated Water Column” sample: • Measure and Record the residual volume left after the chemistry samplings in the surface niskin bottle, because this is the only Carbon sampling depth common to all sites. • Base the collection of the remaining residual volumes, to be added to the 20 litre “integrated” carboy sample, on the measured residual volume from the surface niskin bottle to avoid a volume bias from a particular depth. Use the measuring jugs and funnels made from “native/natural“ plastic for this • Discard from niskins sampled deeper than the surface bottle, anything in excess of the volume equal to that obtained from the full sampling conducted from the surface niskin. • Do not include water sampled from deeper than 50m in the carboy. Phytoplankton pigments – samples from depths equating to moored WQM sensor packs: • There is a requirement for another cast where a niskin of water is collected from each of the 2 depths that correlate with the moored WQM sensors, for pigment analysis (by HPLC) to check WQM pigment performance • As with the water collected for the “Water Column” carboy sample, use the funnels made from “native/natural“ plastic to collect the niskin contents into individual, labelled, 5 litre containers. • Genomic surface sampling for microbial and phytoplankton analyses requires a 3 litre niskin sample collection from the same depths • Remember to keep all collected waters cold/cool and in the shade to minimise sample degradation. • Keep collected waters in carboys and containers quite separate from other samples which may hold any formalin whatsoever • Where and when necessary, preserve or pre-treat samples as outlined in the detailed sampling protocols for each parameter as described below • Endeavour to avoid sample biodegradation by holding these samples from collection time to post-processing, to less than four (4) hours maximum. NRS Sampling Manual Page 14 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL For discrete water samples to be taken, 5L Niskin bottles triggered by messenger (Brass “Go-Devil”) on a single wire cable will be taken at intervals of approximately 10 metres in most cases. Some of these discrete samples will be taken at roughly the same depths as the 2 WQMs for mooring comparison/calibration. NRS moorings will be situated at the same NRS biogeochemical sampling sites. Training in all of the following sampling methodologies is essential prior to undertaking any field work. This should be done under the guidance of an experienced sampler with particular expertise in their area of specialisation. Some of the newer sampling stations have less experienced staff conducting the sampling which is why the annual training workshops are absolutely essential. Field sheets have been designed for each individual NRS to clarify what samples are to be taken at each unique site – in particular the non-standard depths chosen for Carbon sampling. If the use of a laptop is not possible, copies of these sheets will be made on waterproof paper (polymer), taken into the field and filled out fully with a lead pencil or black biro. Remember, for complete and appropriate metadata production, each field on the log-sheet must be filled in for the station being sampled. It is most important for all sampling teams to read and become familiar with what log sheets, forms, notes, records of variation from methods, etc. as described in the Data Handling, Archival and Retrieval section towards the end of this manual, must be carried through when sampling is undertaken at their station. When conducting the sampling at each site, it is recommended that the vessel remain as close as safely possible to the surface mooring buoy in order that the actual samples that will be used to check against the WQM performance are as relevant as possible to what the WQM is measuring at that point in time. There are three important things to bear in mind with the sampling order as laid out in the Suggested Field Sampling Order and on the sampling log sheets for each unique NRS sampling site. These are: • Carbon samples must always be sampled at the surface, with the residual niskin volume measured and then matched from the deeper niskins, in order not to bias the “Water Column carboy” by being made up from differing residual niskin volumes. • If all the biogeochemical sample “water column” depths cannot be gathered in the first collection cast due to vessel limitations, then please choose to do the deeper samples first. • The WQM calibration samples, the water column carboy (<50m) and the accompanying biogeochemical sampling (<50m) must be carried out as close to station departure time as possible in order that the time from sampling to post – sampling treatment is minimised. This is in order to avoid pigment and biota biodegradation with time. Thus, if necessary, the preference for conducting a deeper cast prior to the shallower one. NRS Sampling Manual Page 15 IMOS National Reference Station Field Sampling Secchi Disk Observations Although the disk does not provide an actual quantitative measure of light penetration, the readings (mean) can provide a method to determine limits of visibility for comparative purposes • This is the first task carried out upon arrival at station • A Secchi disk is a weighted disk of 18cm (8 inch) diameter divided into alternating quadrants of black and white, which is lowered over the side on a thin rope with depth intervals marked on it. • If possible, lower the Secchi disk on the lee side of the vessel in order to minimize wind driven surface ripples. • Also, if possible, carry the Secchi deployment on the sunny side of the vessel although this can be difficult due to vessel drift or strong currents. • The disk is lowered until it is just no longer visible. This depth is recorded on the field sheet • The disk is then raised until it becomes visible again and this depth is also recorded on the field sheet • The mean of these two depths is considered to be the limit of visibility • Do not wear sunglasses – particularly polarised lens type - as this will introduce error in the readings • Record the data for Secchi disk depth in metres on the field log sheet NRS Sampling Manual Page 16 IMOS National Reference Station Field Sampling CTD PROFILES After the Secchi disk measurements have been carried out, conduct a profile of the water column using a CTD (with ancillary sensors) – from the surface to 2.5 m from the sonic bottom. The results can be compared to the WQM performance and the hydrology and phytoplankton samples later used for calibration/processing of the CTD data. This will enable a comparative check of the WQM performance over the duration of their moored deployment. Ensure the Fluorometer cap is off the Fluorometer prior to deployment. One thing that can draw attention to the presence of the cap is to colour it bright red. After retrieval of the profiling CTD, check if the CTD cast was successful by connecting the CTD to the laptop provided to each site and check to see if data were successfully obtained. If not, please take the time to repeat the profile. After use for a sampling trip, thoroughly rinse the instrument and cage with fresh water. Also wash the sensors, via the plastic tubing with ~ 500 mL of reagent grade water to waste. Next, leave the sensors and tubing filled with reagent grade water until the next time it is in use. Forward the “raw” and “processed” CTD electronic data to the ARCS staging area: https://df.arcs.org.au/ARCS/projects/IMOS/staging/ANMN all the CTD information: *.con files, *.hex files, *.cnv files accompanied by any other CTD documentation in PDF format. Please see important note in the “data handling section” with information relating to “blocked” files sent as e-mail attachments. For any single sampling, basically, put all data and documents in a non-password locked *.zip file and forward to at CMAR – Hobart or to a FTP site set up for these data (still as a .zip file) Please see a detailed description of the CTD set-up, use, data extraction and data production in the companion documents at the first link on this page: http://imos.org.au/anmndocuments.html NRS Sampling Manual Page 17 IMOS National Reference Station Field Sampling ZOOPLANKTON Sampling Method The zooplankton net will be dropped 3 times at each station: • The first is for dry weight analysis (preserved in formalin as explained below) • The second for taxonomic analysis (preserved in formalin as below) • The third is for genomic analysis which will be later preserved in liquid nitrogen. So the zooplankton sample from the third cod-end should be transferred to a black 1 litre jar (without formalin). Keep this jar in a cool dark place, before being concentrated and scraped into a 5 mL cryovial onshore The zooplankton will be sampled using a robust drop net (Fig. 2; Heron 1982) that does not require a flow meter. The drop net provides a depth-integrated sample from the surface to seabed. The sampler has a standard plankton net attached (e.g. WP2), with a closing collar attached to a weighted ring (about 25 kg) and a rope attached to the collar that can act like a noose. Fig.2 Plankton drop net Heron (1982) NRS Sampling Manual Page 18 • A 100 µm mesh will be used as the IMOS standard to capture the small zooplankton that is common in Australian waters. • Before the first drop, make sure that the net is wet (spray it down with the deck hose), in order that the net is allowed to fall efficiently through the water column and match the following net deployments. This is not necessary on subsequent hauls because the net is already wet • The device is dropped over the side of the boat and falls (of its own accord) at ~1.0 m.s-1. • When the time to reach the desired depth has elapsed, payout of the 12 mm “silver” rope is stopped, thus closing the net. (Note: We will provide the expected elapsed time for when the net will be noosed off, according to the sonic depth at each station) • Timing with a stopwatch should be performed by someone who is not operating the net, and the drop starts as soon as the collar hits the surface • Once the desired time has elapsed, the progress of the rope must be halted so the noose of the net closes. The appropriate way to do this will have to be determined by the equipment available on each vessel and discussion with the zooplankton research leader • The net is usually accurate enough to not require a flow meter (accuracy <3.3% over a drop of 50 m) • The net is hauled up using a winch or pot hauler. It can also be retrieved manually (from shallow water). Because the sample is collected on the way down and noosed closed when the drop is stopped, retrieval speed is not critical • Once onboard, contents of the net are washed down, from the outside of the net and concentrated into the cod-end (sample container at end of net) with seawater from the on-deck hose with a hose fitting. • Reduce the volume in the cod-end as much as possible (by about half by tipping the cod-end to a slight angle so water runs out the netting immediately adjoining cod-end) prior to decanting it into the sample jar • Gently spray bottom of mesh and cod-end to rinse and empty into the sample jars appropriate for the type of sample drop. • For the dry weight and community samples, the sample will be transferred from the cod-end to opaque 500mL plastic jars. • If you need more than one jar from each drop, fill a second jar and label jars A and B • NOTE: In some of the exposed sampling sites where it is found that there is significant vessel movement, prepared small containers holding a measured dose of concentrated formalin (and then contained within another plastic containment jar) has been found to be a satisfactory method of dosing the sample with formalin. Or a preloaded syringe may be used. NRS Sampling Manual Page 19 • Tighten the jars that have been pickled with formalin and then seal around the lid tightly with “duct” tape. • For the genetic analysis sample (3rd drop - net), pour the contents of the codend into the larger black jar provided, tightly cap and place on ice until back on land • Waterproof labels (and pencils for writing) will be provided with the station code and type (dry weight or community) already entered. Date and time should be entered on the labels and placed in the sample jar with sample and formalin. Similarly, write the sample details on the outside of the jars. Be sure to record the station code, the date, time of day and any comments (e.g. strong currents or net hit bottom) in the log book • It is estimated that the net deployment and retrieval, washing down of the net, and preserving the sample will take only a matter of minutes for each sample • If something goes wrong with any drop (e.g. net tangles on way down), rinse the net and cod-end down and re-deploy • A drop net will be provided for each national reference station. If damaged, there are backup nets that can be sent at short notice NRS Sampling Manual Page 20 IMOS National Reference Station Field Sampling SAMPLING WITH NISKIN STYLE WATER SAMPLE BOTTLES Water sampling bottles (Niskin TM style bottles): • The CSIRO Division of Marine and Atmospheric Research uses Niskin style bottles as its primary sampling device to capture discrete water samples from the water column. • In the case of the IMOS National Reference Stations the 5 litre niskins that have been selected for use, have stainless steel internal spring closures. These avoid any toxic effects on phytoplankton collected which have been attributed to latex used as the internal closure band. • NOTE: Due to the heavy amounts of discrete samplings and subsequent treatment of the integrated sample held in the carboy – e.g. for filtration – it will be likely for most stations to conduct a second or even third cast, to obtain the WQM calibration samples and the other water chemistry samples from deeper than 50m – again depending upon vessel capability. Fig. 3 Below: A Niskin bottle (1.7L) in the open, or set, position NRS Sampling Manual Page 21 Fig. 4 Below: an “exploded” view of a niskin bottle to show components that a niskin style water sampler is made up from. Taking the discrete water samples in the water column: • The Niskin bottles should all be stored on the vessel in a rack in the order they will be strung on the sampling wire. Ensure that any reagents to be used for the pickling of samples have been primed ready for use and the tips are clean and show no sign of coloured chemical. • Ensure that the thread on the wire clamp bolts is just slightly “burred” on the end, to avoid the clamping wing nuts from spinning freely off the bolt and over the side into the water. • When using a sampling wire with a weight on the bottom, attach the "bottom", or deepest, Niskin bottle approximately 1.5 to 3 m above the weight and in the open position in order that the bottle can fully flush with water as they are lowered to the sampling depth. • When the bottles are in the open position, check that the top air bleed is screwed firmly closed and that the bottom sample spigot (lower) is in the off position - pulled out fully to the “double-click” position. NRS Sampling Manual Page 22 • Ensure the wire is fully seated in the grooves in the aluminium wire clamping bolt mounts and the steel bolts. Test that the wing nuts are done up tightly. • Pay out enough wire to equal the "next to bottom" position of the next Niskin bottle according to the station log sheet. This will vary for each unique National Reference Station, depending upon: historical reasons; station depth; or correlation to any adjacent moored instruments. • Firmly attach the next bottle to the wire. • Next attach a go devil messenger to the second deepest Niskin bottles’ messenger release pin and then snap the go devil to the wire. This order of attaching the messenger helps avoid loss of messenger or premature triggering (closing) of the niskin below. • Repeat this procedure (including messenger attachment) until all Niskin bottles for the sample cast are fastened on the line in the open position. • If a large vessel uses a CTD rosette in conjunction with niskin bottles for sampling, again ensure all bottles are in the order that they are to be sampled and mount them around the rosette in the open position (again checking the air bleeds and sample spigots are firmly in the off position). • In either case of sampling, allow the bottles to soak at sample depth for a minute or so prior to sending a go-devil messenger down the line to trip (close) the first bottle. A 1 – 2 minute soak/flush of the bottle at sample depth on a cable or rosette has been shown to greatly increase the quality of the sample that is taken on many occasions. • Allow enough time for the subsequent messengers to trip each bottle depth below the first, until the bottom bottle is tripped, before winching the Niskin bottles to the surface. By touching the wire with one hand it is possible to feel a strong “thump” on the wire as each messenger triggers the next bottle. The bronze messengers fall at approximately 60m per minute. • Upon retrieval, when each bottle reaches a safe height to handle, remove the now full water sample bottles and messengers from the wire and place them in order in the rack ready for sampling. • If due to drift, there is a reasonably large “wire angle”, for safety reasons it is advisable to fasten a stainless “open hook” to a “comfortable length” of rope tied off to the vessel rails, which can be easily slipped around the cable below the niskin to hold the cable close and more upright for the sampler as the bottles are removed. • It is critical to note and record if there are any leaks from the bottles upon retrieval – e.g. end-cap not seated correctly, end–cap accidentally knocked open, leak from lower sample spigot, etc. – as this may affect the eventual integrity of the samples from a particular bottle. NRS Sampling Manual Page 23 IMOS National Reference Station Field Sampling COLLECTION OF WATER CHEMISTRY SAMPLES Water Chemistry Sampling Order (suggested) • This may vary depending on the practicalities of sampling at different station. • Samples can be drawn from the Niskin bottles that have been retrieved and placed securely in the niskin rack. • Niskin bottles are also always sampled from deepest to shallowest as the deeper samples have undergone the greatest change in temperature and pressure. Dissolved gas samples should always be taken first As a hint – when sampling for dissolved gases – keep the silicon tube that is used, wetted prior to use and this will assist in minimising bubble formation in the sampling tube • Dissolved Oxygen is usually the first sample to be drawn from the niskin bottle (however this is only being sampled at 2 NRS stations) • Because the carbon parameters are also dissolved gas dependant, the TCO2 (Total Dissolved Inorganic Carbon) and TALK (Total Alkalinity) are the next – or in all but 2 NRS cases, first – sampled. • Due to Niskin bottle volume limitations the next sample taken is salinity. • The last of the water chemistry samples to be drawn from the Niskin bottles are for nutrient analysis - nitrate/nitrite, orthophosphate, silicate and ammonia • Good sampling technique is the single most important factor in producing accurate Dissolved Oxygen and Carbon data. After the last of the water chemistries has been sampled from the niskin bottles, the residual water in the bottles is collected to make up the “composite/water column” sample; the description of which follows the detailed explanations of the chemistry sampling techniques described next. NRS Sampling Manual Page 24 IMOS National Reference Station Field Sampling COLLECTION OF WATER CHEMISTRY SAMPLES Drawing the water chemistry samples Dissolved Oxygen • Dissolved gas samples are taken as soon as possible after sampling. • Before each sample is collected, each Niskin must be checked for leaks and potential contamination. Open the spigot (lower tap) and watch carefully for any water flow. If the Niskin leaks, then air is entering the Niskin. Samplers should record this information on the log sheet for later reference by the analyst and data processor. Recollect the sample if possible. Attach a 20 cm length of silicon tubing over the spigot of the Niskin. Start the water flow by opening the air bleed on the Niskin. Allow a small volume to run to waste, and use this to clear the tubing of bubbles. Air bubbles may stick to the sides of the tubing. Squeezing, flicking or manipulating the tubing should remove them. • • Stop the flow of water by pinching the tubing close to the spigot. Hold the 150 mL sample bottle at an angle of approximately 45 degrees. Lower the tubing into the bottom of the bottle, and slowly release the tubing so that water flows gently into the bottle. Take care to minimise turbulence, to avoid introducing atmospheric oxygen into the sample. • When the bottle is about a third full, pinch the tubing closed and discard the water. Repeat the rinse twice. Keeping the tubing in contact with the bottom of the bottle at all times, slowly release the tubing until the water flows freely and fills the bottle but with minimum turbulence. Allow at least twice the volume of the bottle to overflow. • Slowly pinch off the tubing to reduce the flow rate, and remove the tubing from the bottle. There should still be enough water flowing to ensure that the bottle is full to the brim when the tubing is completely removed. • Without capping the bottle, quickly proceed to the pickling reagents. • Use gloves and safety glasses when using these reagents and wash with copious amounts of water if skin or eye contact is made • Immediately add 1.0 mL of reagent 1 (3 mol/L Manganese chloride) by placing the tip of the reagent dispenser below the surface of the sample and pumping the dispenser once. The tip should extend below the neck of the oxygen flask so that the flocculent does not form in the excess seawater in the neck of the flask. This water has been in contact with the atmosphere, and could result in sample contamination. • Straight away, add 1.0 mL of reagent 2 (4 mol/L sodium iodide/8 mol/L sodium hydroxide) in the same manner. The reagents are very much denser than seawater and will sink when added, displacing that portion of seawater that has been directly in contact with the atmosphere. • The sample should now be stoppered so that no air bubbles are trapped in NRS Sampling Manual Page 25 the bottle. Try dropping the stopper from a height of about 2 cm above the bottle. Inserting the stopper slowly is more likely to trap a bubble. It is imperative that no bubbles are trapped in the bottle, as they will completely invalidate a sample. If you suspect a sample for any reason, throw it away, rinse the bottle thoroughly and repeat the procedure. Note this down on the log sheet for reference by the analyst. • Shake the bottle thoroughly at this stage to completely form the flocculent and disperse it evenly throughout the bottle. The shaking is critical, and should involve a snapping motion of the wrist, where the bottle is completely inverted rather than just shaking. Invert the bottle in this manner at least 20 times before placing the bottle in the box. The samples are light sensitive at this stage, and the lid should be left down at all times. • Flood the flared neck of the bottles (around the stopper) with water to prevent air being drawn into the samples. This is particularly important if there is to be a lag time between sampling and analysis or a large temperature difference between the sample and ambient temperatures occurs (particularly in tropical, warm waters). (Critchley, G.; personal communication). • The sample bottles should be shaken a second time in the laboratory to ensure thorough mixing of the contents and allowed to re-settle. NRS Sampling Manual Page 26 IMOS National Reference Station Field Sampling COLLECTION OF WATER CHEMISTRY SAMPLES Carbon Sampling is conducted at varying depths for each specific NRS This is the first sampling conducted, at those stations where chemical Dissolved Oxygen sampling is not carried out, and the site specific depths have been selected by the Carbon Group. Kits and pre-numbered bottles for Carbon sampling will be forwarded to each site prior to sampling on board the vessel. The kits can be retained at the on-shore component of the station after returning from sampling, for later transport to CMAR, Hobart. As previously mentioned, the Carbon samples are dissolved gas dependant, therefore are the first samples which should be taken. These samples are for Total Dissolved Inorganic Carbon (TCO2) and Total Alkalinity (TALK), according to the Carbon groups’ sample collection protocol (See next). NRS Sampling Manual Page 27 IMOS National Reference Station Field Sampling CARBON SAMPLING Two types of sample are required for carbon dioxide measurements: • TCO2 (total dissolved inorganic carbon) and • TALK (total alkalinity) TCO2 bottles are square with black lids and TALK bottles are round with blue or white lids. They are supplied in strong boxes for safe transport. Please fill in the log sheets provided and label the bottles before sampling with the date, time, location and depth. Labelling tape is provided. Sampling Total Dissolved Inorganic Carbon Carbon dioxide is a gas dissolved in seawater and is sampled as soon as possible after opening the niskin bottle, usually immediately after dissolved oxygen (DO) sampling. 1. 3. Fit the flexible end of a sampling tube (provided, or you can use the DO sampling tube if it is long enough) over the bottom outflow valve of the niskin. 2. Start flow through the tube by pushing in the outflow valve. At this point carefully note if there is any water flowing from the sample spigot. If there is, note it on the field sheet as a possible leaking niskin bottle. To commence the niskin sample flow, open the upper air bleed and the water should now commence to flow freely from the sample spigot. If there are air bubbles in the sampling tube pinch and release a few times to remove them. Pinch the flexible part of the tube to stop the water flow. Insert the tube to the bottom of the sample bottle and slowly release pressure on the tube to allow water to flow. Fill the bottle to 1/3 full, swirl slightly and invert the bottle to pour the contents out over the sampling tube to rinse both bottle and tube. Do this 3 times. Pinch the tube again then release carefully, trying to avoid creating turbulence in the bottle so as to minimise gas exchange with the sample. 4. Fill the bottle (with the tube still at the bottom) and allow it to overflow with about half the volume of the bottle. Pinch the tube to stop flow and withdraw it carefully from the bottle. The level of sample in the bottle should be as shown in this picture. If it is too full, pour out a little water. NRS Sampling Manual Page 28 5. Using the pipette provided add 100μL of saturated mercuric chloride solution to the bottle. Keep the pipette tip just above the sample surface. CAUTION: mercuric chloride is toxic. Wash with copious amounts of water if it touches your skin. Screw the cap on tightly and shake the bottle 4 times to distribute the mercuric chloride. 6. Store samples at room temperature in the box provided, and retighten the lids after an hour or so. Sampling Total Alkanity Alkalinity is sampled immediately after TCO2 samples are collected. 7. Use the same technique as for TCO2 sampling. Tip out some water after withdrawing the sampling tube so that the level is as shown here. 8. Using the pipette provided add 100μL of saturated mercuric chloride solution to the bottle. Keep the pipette tip just above the sample surface. CAUTION: mercuric chloride is toxic. Wash with copious amounts of water if it touches your skin. Screw the cap on tightly and shake the bottle 4 times to distribute the mercuric chloride. 9. Store samples at room temperature in the box provided, and retighten the lids after an hour or so. NOTE: • All TCO2 AND TALK samples must be returned to Hobart in the boxes provided where ALL TCO2 AND TALK SAMPLES TAKEN, WILL BE ANALYSED Contact information for any queries regarding Carbon sampling: Kate Berry CSIRO Marine & Atmospheric Research Castray Esplanade Hobart Tas 7001 Phone: (03) 6232 5270 (W) Phone: (03) 6227 9589 (H) Email: kate.berry@csiro.au NRS Sampling Manual Bronte Tilbrook CSIRO Marine & Atmospheric Research Castray Esplanade Hobart Tas 7001 Phone: (03) 6232 5273 (W) Phone: (03) 6267 1339 (H) Email: bronte.tilbrook@csiro.au Page 29 IMOS National Reference Station Field Sampling COLLECTION OF WATER CHEMISTRY SAMPLES SALINITY SAMPLING • In the case of routine IMOS hydrochemical sampling, the next parameter to be taken from the Niskin bottle is salinity. • As with all sampling, the field sheet should be regularly referred to in order to ensure that the correct sample bottle is being used for the appropriate Niskin bottle. • Salinity sample bottles (250 mL amber glass bottles with Teflon lined caps) will contain old residual sample from a previous analysis when they are to be used next. This is done to prevent drying out of the bottles and contamination by salt crystals, as well as providing an initial rinse to remove any salt build-up. • Great care must be taken when sampling for salinity to avoid contact of the bottle with the sample spigot and to minimise contact between the sample spigot and the hands. • Shake the salinity bottle with old residual sample in it, and then decant the old sample over the cap of the bottle and around the screw neck of the sample bottle. This will remove the bulk of the salt built up since the last sampling and allow better rinsing with the new water. • Without touching the sample spigot to the sample bottle take approximately one quarter of a bottle of water, loosely cap the bottle and shake vigorously. Pour this rinse to waste over the salinity cap, neck of the salinity bottle and over the sample spigot. The sample spigot is rinsed to avoid contamination from surface water, rainwater, and any other source. • Repeat this rinsing procedure another two times. Close the spigot in between rinses to avoid niskin sample waste • Fill the salinity bottle to the shoulder (approximately 3 cm from the top of the bottle) - to allow for expansion of cold samples. • Tightly cap the sample bottle, return to the crate and prepare for the next sampling. • Place the crate of salinity samples in a cool dark place until they are shipped to the salinity laboratory. Upon arrival at the laboratory, allow them to reach ambient in the controlled temperature salinity laboratory (approximately 12 hours). NRS Sampling Manual Page 30 IMOS National Reference Station Field Sampling COLLECTION OF WATER CHEMISTRY SAMPLES NUTRIENT SAMPLING • Nutrients are sampled last of all for the hydrochemical sampling. The samples are taken in triplicate in single use polypropylene tubes with screw caps. Ensure the tubes have the correct labelling for the niskin bottles to be sampled from. • As with all samples, draw the nutrient samples from deep to shallow. • Taking good nutrient samples is similar to that technique employed for the sampling of salinities. • Do not touch the tubes to the sample spigot and ensure your fingers do not contact the inside of the tubes or the caps. • Make sure no one is smoking in the general vicinity and avoid engine fumes (important for ammonia sample). • Match the triplicate nutrient tubes to the correct Niskin bottle and open the sample spigot. Half fill the tubes with sample water, loosely cap the tubes and shake in order to rinse the inside of the tubes and caps. • Discard contents. • Fill the tubes to the 10 mL mark and no more. • If more than 10 mL is sampled, discard the excess and tighten the caps. • Freeze the sample tubes – preferably in the upright position. • Freeze the samples in an upright position as soon as possible using a freezer if available (that has had no biological samples previously stored in); or keep cool with a couple of pre-frozen “ice-packs”, until they can be frozen in a clean freezer • If the nutrient tubes are over filled and frozen, the sample in the tube separates into brine/freshwater layers with the onset of freezing and can force the nutrient rich brine to seep out the top of the tube creating significant errors. (Critchley, G.; personal communication). NRS Sampling Manual Page 31 IMOS National Reference Station Field Sampling COLLECTION OF A DEPTH INTEGRATED CONTAINER (Carboy) FOR A VARIETY OF PARAMETER PREPARATIONS CARRIED OUT ASHORE • Measure and Record the residual volume left in the surface niskin bottle, because this is the only Carbon sampling depth common to all sites. By basing the collection of the residual volumes to be added to the “integrated” carboy sample from the other niskins (< 50m), on that left over from the surface niskin bottle, it will avoid biasing the “integrated” carboy sample. This measuring step avoids introduction of a greater residual water volume from another niskin not sampled for Carbon. • The 20 litre carboy, tap, measuring jugs and funnels should all be of “native/natural“ plastic – not coloured – and thoroughly cleaned by soaking in reagent grade water and occasional shaking, for a week prior to first use. • Decant the entire residual contents of the surface niskin, after the water chemistry sampling into a 3 – 4 litre graduated “native/natural“ plastic jug. Measure as accurately as possible and record the volume before transferring the water into the plastic carboy via a large “native/natural” plastic large mouthed funnel. • Repeat this process for the remaining niskin bottles until all bottles sampled at <= 50m are ready. Of course, some sites are only some 20m deep, in which case repeat bottle collections – which are measured but not necessarily sampled for chemistry requirements – would have to be taken until the required volume of 18 – 20 litres is obtained. • Store the “composite/water column sample” carboy in a cool/cold (dependant on sampled temperature), and dark location (under wetted shade cloth is an option), until sub sampled on shore. If sampling in shallow water e.g. 25m, then it may be necessary to take 2 x 5L at each depth - or equivalent, to make up the required volume for the subsequent sub sampling and filtration from the carboy. • The minimum volume that is required to be collected in the composite sample carboy is 13-14L (less at tropical stations) as there are a number of different analyses required from this carboy. • From stations with depths below 50 metres please sample as laid out in table 4 above, unless the vessel is capable of niskin sampling to the bottom in which case a single cast, to the bottom may be an option to consider. • Discard from niskins sampled deeper than the surface bottle, anything in excess of the measured volume equalling that remaining after the full sampling carried out from the surface niskin. NRS Sampling Manual Page 32 IMOS National Reference Station Field Sampling COLLECTION OF WQM PHYTOPLANKTON CALIBRATION SAMPLES • Phytoplankton (pigments) samples, to check the sensor performances from depths equating to the (normally only 2) WQM sensor packs are required to be sampled at each NRS site. • The water chemistries will have already been sampled from the depths correlating to the WQM packs, so these 5 Litre samplings are to be conducted only for HPLC pigment analysis • Like the water collected from the 5 litre niskin bottles for the “depth integrated” carboy sample, when transferring the collected water – which is not necessary to measure for volume – a large funnel and 5 litre “native/natural“ plastic containers should be used. • Also, like the water collected for the “integrated “carboy, these items should be thoroughly cleaned by soaking in reagent grade water and occasional shaking, for a week prior to first use. • Once the water correlating to the WQM sensor packs has been sampled and stored, ensure that they are stored in a shady, cool spot the same as the integrated sample carboy and kept well away from any formalin or formalin preserved samples. • Also, like the water collected in the “integrated” sample carboy, retention time in containers from the time of sampling to the time of the on-shore, post-sampling sample treatments, must be kept to an absolute minimum due to the potential for biodegradation of the sample. • This is why, as mentioned earlier, if sampling casts are to be done on a vessel that requires 2 casts to complete the required depth coverage of a particular station, conduct the deeper cast where “living organisms” will not degrade the sample type taken at depth first; and then carry out the shallow “photic” sampling cast second, with the WQM and microbial samples taken last – just prior to departure from the station to carry out the further sample treatments ashore. NRS Sampling Manual Page 33 IMOS National Reference Station Field Sampling Protocol for IMOS/AMMBI microbial sampling (revised April 2012) AMMBI = Australian Marine Microbial Biodiversity Initiative, in which IMOS is one of the collaborators with CSIRO and Bioplatforms Australia. • At the initiation of the discussions of which parameters would be achievable and desirable for routine collection and analysis and for subsequent inclusion in this manual; Genomics/molecular chemistry/genetics was and still is a rapidly evolving area of science and probably particularly in the domain of Marine Science. • For example the volumes required 2-3 years ago, for filtration and subsequent analysis were quite large – in the vicinity of 100+ litres onto filters with an approximate diameter of 145mm. Techniques have rapidly evolved now to the point where a volume of 2L, filtered onto a sterile filter is now adequate to carry out the microbial analyses. This now is an achievable and realistic target for the smaller vessels undertaking the sampling for the IMOS NRS Biogeochemical sampling. On station: 1. Conduct Niskin bottle cast(s) to pre-determined depths (x6). Make cast as close to last activity on station as possible. 2. For each depth, rinse funnel and container (labeled with relevant depth) with retrieved seawater (~1 L). Discard. 3. Fill container with a minimum of 3 L seawater and store in Eski. Repeat for all depths. 4. Make sure Eski internal temperature stays relatively close to sample temperature until sample processing. • Like the water collected from the 5 litre niskin bottles for the “depth integrated” carboy sample, when transferring the collected water it is not necessary to measure for volume. A large funnel and 5 litre “native/natural“ plastic containers should be used. • When the water samples have been collected and stored in their 5 litre “native plastic” containers, ensure that they are stored in a shaded, cool spot the same as the other carboys and kept away from any formalin or formalinpreserved samples. At northern sites, store in a coolbox. • The Genomic carboys are transported with the other carboys to the onshore facility for the post-sampling treatment. NRS Sampling Manual Page 34 IMOS National Reference Station Field Sampling Safety Considerations when conducting sampling on Small Vessels and during post – sampling treatment and preservation In order to avoid any potential injury to Personnel during the field sampling and the onshore post – sampling treatment and preservation, ensure that the following points are adhered to: When conducting a Sampling excursion we have determined that in order to carry out the work safely and efficiently it is essential that there be a minimum vessel crew of three (3): • A certified coxswain (minimum requirement) or skipper • 2 persons wholly dedicated to undertaking the sampling requirements • One of the three staff needs to be licensed to drive a heavy vehicle, if the vessel requires trailering to and from a NRS departure and arrival point. If, when conducting the sampling there is enough vessel drift which leads to a large wire angle and reaching for the wire is uncomfortable or dangerous, it is recommended that an open hook attached to a short length of rope fastened securely to the rails be used to hold the wire/cable at a comfortable reach and as close to vertical as possible. When slipping the hook around the cable during a niskin cast, do so below the bottle in order to avoid accidently knocking open an end cap of the bottle, causing sample contamination or loss. Personal kit should include, at the least: • Steel capped boots or shoes • A self-inflating safety vest • A personal EPIRB unit • Gloves If the coxswain or skipper decides that conditions are not safe to conduct the work then they can, without blame, cancel sampling at any stage of the excursion. Observe safe handling of concentrated formalin (zooplankton samples). Observe safe handling of mercuric chloride (Carbon samples). Observe safe handling of Lugols solution during the post – sampling treatment and preservation Observe safe handling of Glutaraldehyde during the post – sampling treatment and preservation Observe safe handling of Liquid Nitrogen and Dry Ice during the post – sampling treatment and preservation Carry laminated mini-MSDS sheets for all potentially hazardous materials, on all sampling excursions. NRS Sampling Manual Page 35 IMOS National Reference Station Field Sampling POST SAMPLING ACTIVITIES There are a number of post sample collection activities which must be undertaken upon return to shore Post-sample preparation has the utmost priority as the first action • Sample transport to the shore based facility for further sample treatment should be carried out as a priority. Whilst some samples are already preserved and long term stable, many need further preparation prior to being prepared for storage and transport to the analytical laboratories. Bear in mind, many of these are subject to irreversible biodegradation. • Prior to departure, maybe even the preceding day, it is wise to set up all the sample treatment equipment – measuring cylinders, vacuum pumps, filtration apparatus, etc in order to ensure a rapid start can be commenced on the samples. Don’t set up filter papers early as they don’t absorb. • Post sampling treatment is dealt with shortly, however it is essential that the completed field logsheet and its accompanying post-sampling treatment sheet are not forgotten; and accompany all the samples whilst in transit to the shore treatment facility Essential equipment maintenance Vessel and winches, etc: • Mainly being small vessels undertaking the field operations, they are best to have all components, motors, etc washed clean with fresh water as soon as is practicable. Zooplankton gear: • After each sampling trip, please rinse the entire net and netting with freshwater, dry in the shade and store out of the sun. Sampling gear – niskin sample bottles and messengers: • At the conclusion of a sampling trip, remember to rinse the niskin bottles with fresh water, inside and out, whilst in the cocked/open position. Leave to dry in a clean environment for approximately 3 days before closing the bottles until their next use • Also wash off the niskin bottle messengers with fresh water and store them in an “open and airy” position or container in order for them to thoroughly dry. If this is not carried out, the messengers will become coated with verdigris and become very stiff and awkward to use. NRS Sampling Manual Page 36 Sampling gear – secchi disk: • Wash down the secchi disk and rope with freshwater to maintain it in good condition. Again, allow the fish box container open for a while to allow the contents dry out Sampling gear – Seabird CTD: • Ensure that the Seabird CTD’s and protective steel frames are washed down with fresh water, and the detector units are rinsed with reagent grade water (deionised is alright, but not quite as good). The sensors are stored wetted as recommended by the manufacturer to help eliminate the chances of calibration changes caused by a continuing wet and dry cycle. In shared equipment storage areas, ensure that as it was requested at the outset, the IMOS NRS Biogeochemical equipment is not only clearly labelled but kept in a large container/skip of some kind in order that the items are not used by others who may damage your gear and not tell you about this having happened. Once the sampling gear has dried, the containers may also have the lids fastened firmly. NRS Sampling Manual Page 37 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Essential Sample Coding Unique sample identification coding: In order to identify a specific sample, please use the following when labelling sample storage containers either at the time of collection or during the following period of post – collection sample processing : NRSSSSYYYYMMDDddd where: NRS denotes National Reference Station SSS denotes the 3 ALPHA IMOS NRS code (e.g. MAI for Maria Island), YYYYMMDD denotes 4 digit year, 2 digit month, and 2 digit day/date, ddd denotes a 3 digit record of a discrete sample depth OR for integrated water column samplings (carboy and drop net) please use: NRSSSSYYYYMMDDWC where: NRS denotes National Reference Station SSS denotes the 3 ALPHA IMOS NRS code (e.g. MAI for Maria Island), YYYYMMDD denotes 4 digit year, 2 digit month, and 2 digit day/date, WC indicates an Integrated Water Column sampling Please note: There is no longer a need for recording “sample trip number” (ss) as included in the original coding, as this requires prior knowledge which may be overlooked, misread, forgotten, etc….. and can lead to possible confusion. It was originally included for ease of tracking the number of sampling trips per annum. All date and time codes must be recorded in UTC – whether on a field or filtration log, file name or in CTD configuration and recorded time. TTTT = Time in 24 hour clock notation only please • In order to distinguish similar sample types taken or prepared for storage and later shipment (for example: different phytoplankton filters for pigment analysis from different sources taken during a single NRS sampling excursion and stored in cryovials); the following information must also be noted on the container the sample is stored in. It is a means of actually denoting sample TYPE and/or ORIGIN. • This notation is required only for those samples treated during the after sampling treatment phase. So it does not include Salinities, for instance. NRS Sampling Manual Page 38 • ZOOPLANKTON SAMPLES (WC): From each NRS sampling trip there are 2 plastic (sealed jars) preserved with formalin. Please note on the jars: ZOOWCdn where: ZOO = Zooplankton; • WC = “Integrated” Water Column; dn = Drop number PHYTOPLANKTON SAMPLES (WC, WQM – deep, WQM – shallow): PHYNUMWC Preserved with Lugol’s iodine for Counts and Community / species composition and estimated phytoplankton biomass PHYPIGWC Filters in cryovials in liquid Nitrogen for HPLC pigment composition from the composite (Water Column) sample PHYPIGWQMS Filters in cryovials in liquid Nitrogen for HPLC pigment composition from the WQM (Shallower) PHYPIGWQMD Filters in cryovials in liquid Nitrogen for HPLC pigment composition from the WQM (Deeper) PHYCYTWC 3 cryovials preserved with Glutaraldehyde for Flow Cytometry • HYDROCHEMISTRY SAMPLES: SUSMATWC • 3 values each site (Total, Organic, & Inorganic) plus blanks, stored in a “petri slide” in a cool dark position GENOMICS SAMPLES (nomenclature to be checked): GENZOOWC A strained sample preserved in a cryovial for Zooplankton Genomics GENMICddd A sample from each nominated depth on a filter in a centrifuge tube / snaplock bag for microbial genomics • Transport requirements from each NRS to the points of analyses are covered for each sample type, in a combined transport section later in this document NRS Sampling Manual Page 39 IMOS National Reference Station Field Sampling Post field collection sample preparation • PLEASE NOTE: The on-shore preparation of some samples within this manual need to be stored in cryovials in liquid Nitrogen Dewars. Groups sending samples in liquid nitrogen must use the proper cryovials – external screw thread with silicon seal. It has been identified that “pop-on” style lids can become projectiles when they are removed from the liquid nitrogen and thus a potential OH&S issue will be avoided. Each site has been provided with the approved/ recommended type – 2mL volume tubes for pigment/HPLC samplings and 5 mL tubes for zooplankton Genomic determinations. ZOOPLANKTON • Genomic zooplankton sample treatment • Once back on land, pour the “cool blackened jar” sample through the fine mesh screen and concentrate sample to one side using a squirt bottle of water. • Next rinse lightly with distilled water, and scrape sample out of the mesh container using a metal spatula to avoid contact with any organics. The process of light rinsing to collect the sample against the side may have to be repeated in order to obtain the entire sample. Pressing a paper towel under the mesh may assist in pulling water through gelatinous samples. Eliminate as much water as possible. Don’t fill above the white line on tube. • Place the entire collected zooplankton sample into a clearly labelled 5 mL cryovial(s), use a cryopen to label according to the coding described in the sample coding section, above. If large salps or jellies are present, remove and place in a 50mL specimen jar and record number of containers used. • Next, place the cryovial/s in liquid nitrogen, ensuring that you are wearing protective gloves and safety glasses/face shield. • If the treatment is carried out at a “remote site”, place the cryovial in a dry shipper (it will freeze the cryovials and keep frozen for up to 10 days). Ship them to the central storage Dewars and transfer to the storage Dewar wearing protective gloves and safety glasses/face shield. • Store the cryovials in liquid nitrogen storage Dewars provided for each Central site, ensuring that you are wearing protective gloves and safety glasses/face shield. Periodically transport via “dry shipper” Dewar within the provided freighting travel cases. NRS Sampling Manual Page 40 IMOS National Reference Station Field Sampling SAMPLE TREATMENT PROTOCOLS FOR THE DEPTH INTEGRATED CONTAINER Sites will require a 240V heavy duty variable rate vacuum pump with gauges, a catcher vessel (10L bottle or flask or similar) between the pump and the filtration apparatus, and a filtering kit with at least 2 filter holders, of preferably 47 mm diameter. The filtration units supplied hold 4 filtration units of 47 mm diameter, allowing for multiple filtrations to be carried out simultaneously thus minimising processing time during this phase. Introduction to the sample preparation for the following analyses: • • • • • Total, organic and inorganic Suspended Matter (SUSMAT) Phytoplankton analysis (PHYNUM) Phytoplankton Pigment Analysis by HPLC (PHYPIG) Flow Cytometry analysis of picoplankton (PHYCYT) Genomic analysis of microbial / phytoplankton populations from discrete depths (GENMIC) Here is a short reiteration of the field collection method for a depth integrated sample collected on site: • At the sample site, after carbon and hydrochemistry samples have been taken from the niskin bottles, the residual niskin volumes – based upon that of the surface niskin after sampling - will be measured and combined into a 20 litre carboy as described. • Catch and measure the residual volume collected from the surface niskin. Record the measured (residual) volume approximately equivalent to that from the surface niskin, which is transferred from each of the other niskins, after chemical sampling, into the 20 litre carboy. Water from any depth greater than 50m is not included in the carboy. • The 20 litre carboy will need to be transported from sampling site to shore in a cooled / shaded container (do not over-cool tropical waters). An esky with a freezer brick or a couple of layers of thick shade cloth, frequently wetted with seawater is ideal. • Endeavour to avoid keeping the samples in carboys for too long as they are required to be processed on-shore as soon as possible after sampling in order to minimise any biodegradation ruining the samples in the integrated carboy and WQM sample containers. NRS Sampling Manual Page 41 IMOS National Reference Station Field Sampling Post field collection sample preparation and treatment Sampling on shore from the composite (water column) sample will require: • Prior to sub sampling the carboy (depth integrated sample) on shore, mix the carboy contents gently but thoroughly, prior to sub sampling as follows: • 1 - 4 litres (depends on location - tropical vs. temperate) for Suspended Matter (duplicate samples of 1 - 4 L each) • 1 litre for phytoplankton species identification • 4 litres for phytoplankton pigments (also see WQM carboys) • 3 mL for flow cytometry (triplicates) Suggested order of processing: Suspended matter duplicates, pigments (WC and WQMs), Lugol’s phytoplankton, flow cytometry, zooplankton genetics sample. It is essential to note the following whilst conducting filtration or treatment of samples taken from any of the waters held in any carboy: • When using the vacuum pump, the pressure should not exceed 5 inch Hg or approx. 100 mm Hg. • Keep a close watch on the level in the catcher vessel - it may need to be emptied before filtering is completed • Filter all samples under subdued lighting where possible. NRS Sampling Manual Page 42 IMOS National Reference Station Field Sampling Sample and pre-treat in the following order: • Suspended Matter (note: procedure under revisison mid-2012) • Using clean stainless steel forceps place a prepared uniquely identified 47 mm GF/F filter in the filter unit. These will be supplied by a laboratory analyst in a numbered petri-slide. • Before filtering the WC sample obtain a “blank” by filtering 2 litres of MilliQ water through a prepared filter. Rinse the sample with 100mL of MilliQ water. • It is important that the original composite sample is well mixed by gently inverting the carboy several times before taking the 1- 2 litre sample. • This composite sample needs to be filtered first to prevent physical and biological degradation leading to an incorrect value. • Place the sample filter on the filtration base and wet the perimeter with a little reagent grade water in order to minimize seawater intrusion at the edge. Attach the top “sample funnel” firmly, and then proceed to filter the sample through the prepared filter. • Measure the volume of sample filtered and record it on the log sheet together with the number of the filter being used. Number is on petri-slide. • You will have to change this volume if you end up not filtering the entire sample. • After the sample has finished filtering, gently rinse the filter with approximately 100 mL of MilliQ water to remove any traces of salt from the filter paper. Pour MilliQ around funnel, not directly on filter. • Remove the filter from the filter unit, with vacuum still applied, using clean stainless steel forceps and return it to the numbered petri-slide that the filter came from initially. • As these filters are pre-weighed and pre-treated it is very important that the entire filter is returned. If the edge starts to separate from the rest of the filter, just make sure all pieces of the filter end up in the correctly numbered petrislide. • Store the filter flat in its petri-slide in a fridge or other cool and dark environment. It is not necessary to freeze these samples. Store in the fridge or keep cool until shipped to Hobart for analysis by the hydrochemistry group. • For analysis, the sample and blanks must be returned to the same laboratory that provided the pre-weighed filter, as all weighing must be carried out using the same balance that was used for the initial preparation of the filters. NRS Sampling Manual Page 43 IMOS National Reference Station Field Sampling • Phytoplankton pigments determined by HPLC from the depth integrated water column sample: • Using stainless steel forceps place a 47 mm GF/F filter in the filter unit. Check that the bottom and top parts are screwed together correctly, as threads seem to cross easily. • 4 litres, or less if the water is particularly turbid, will be filtered through the 47mm GFF filter. • Record the volume filtered on the provided log sheet. • When finished, fold the filter in half using clean flat-blade forceps, with the coloured surface on the inside. • Avoid touching the sample on the upper surface of the filter or touching the filter with hands. • Using clean flat blade forceps, fold the filters into halves/quarters and fold or roll to fit into a clearly marked 2mL cryovial. • Label each cryovial, using the special cryo-marking pen, according to the coding described in the sample coding section, above. • If cryovials are unavailable then as a last option the folded filter can be wrapped in aluminium foil, labelled and frozen in liquid nitrogen. • All details of the sampling, filtration and comments that relate to each cryovial must be recorded on the post-sampling treatment log sheet. • When filtering more than one HPLC sample - such as those sampled at the discrete WQM depths - the cryovials can be placed in a small foam esky containing ice or a freezer block until all pigment samples from that station have been filtered, and then transfer the cryovials to liquid nitrogen, wearing protective gloves and safety glasses. • The liquid nitrogen stored samples are long term stable, stored in a storage Dewar which is maintained with liquid Nitrogen top-ups. These samples can then be shipped quarterly using dry-shipper Dewars and the supplied dry shipper freighting cases, to Hobart for analysis. NRS Sampling Manual Page 44 IMOS National Reference Station Field Sampling Sample treatment protocols from the WQM comparison carboys. • HPLC pigments for WQM sensor comparisons (2 depths) • The WQM calibration checks are from the niskin water that was collected on a separate cast, taken from depths correlating to the 2 moored WQMs and stored in 5L native plastic containers • Follow the above HPLC sample preparation for the niskin sampling that was taken from ~20m (for most NRS sites) - the shallower WQM sample. • Using stainless steel forceps place a 47 mm GF/F filter in the filter unit. Check that the bottom and top parts are screwed together correctly, as threads seem to cross easily. • 4 litres, or less if the water is particularly turbid, will be filtered through the 47mm GFF filter. • Record the volume filtered on the provided log sheet. • When finished, fold the filter in half using clean flat-blade forceps, with the coloured surface on the inside. • Avoid touching the sample on the upper surface of the filter or touching the filter with hands. • Using clean flat blade forceps, fold the filters into halves/quarters and fold or roll to fit into a clearly marked 2mL cryovial. • Label each cryovial, using the special cryo-marking pen, according to the coding described in the sample coding section, above. • If cryovials are unavailable then as a last option the folded filter can be wrapped in aluminium foil, labelled and frozen in liquid nitrogen. • Repeat the HPLC filtration procedure for the deeper WQM - bottom + 2.5m (for most NRS sites) that was sampled from the lower 5 litre niskin bottle. • Ensure to label each cryovial, using the special cryo-marking pen, using the coding method above • All details of the sampling, filtration and comments that relate to each cryovial must be recorded on the post-sampling treatment log sheet. • When filtering more than one sample, the cryovials can be placed in a small foam esky containing ice or a freezer block until all pigment samples from that station have been filtered, and then transfer the cryovials to liquid nitrogen, wearing protective gloves and safety glasses. • The liquid nitrogen stored samples are long term stable, stored in a storage Dewar – maintained with liquid Nitrogen top-ups. These samples can then be shipped quarterly using dry-shipper Dewars and the supplied dry shipper freighting cases, to Hobart for analysis. NRS Sampling Manual Page 45 IMOS National Reference Station Field Sampling • Phytoplankton • Microscopic phytoplankton study. The samples collected for this are preserved using Lugol’s solution • Preparation of Lugol’s solution. • Preparation of acidified Lugol’s solution should be carried out in the laboratory servicing each NRS (again to avoid shipment of chemicals) using the following technique: • The solution requires - 100 g potassium iodide, 50 g iodine, 1L distilled water and 100 mL glacial acetic acid • Dissolve potassium iodide in distilled water, add iodine into the KI solution and dissolve. Slowly add the acid to the solution. • Store the made up Lugol’s solution in a glass container. • NOTE: Users should use Lugol’s solution with concentrations as specified in the recipe above for acidified Lugol’s. Acidified Lugol’s is available through Rowe Scientific: Product CL1252. Commercially available microscope grade Lugol’s may also be used (e.g. Sigma 62650) as long as it conforms to the proportions in the recipe; it may need to be acidified before use, by adding glacial acetic acid to 10% of volume. • Preserving the sample • 1 litre of sample water from the carboy will be preserved as soon as possible, with 5 mL of Lugol’s iodine solution, dispensed via a catalyst dispenser with a cap. • The plastic sample bottle, preferably a “PET” Kartell square, widemouthed 1000 mL 613 bottles is capped firmly and then gently rocked (NOT inverted) 2-3 times to mix the sample and preservative. • The Lugol’s preserved samples should be sealed around the cap and neck with duct tape and clearly labelled with a permanent markeraccording to the coding described in the sample coding section, above • The sealed sample bottle is then stored in a black storage bin or similar in a cool environment until shipment to Hobart. NRS Sampling Manual Page 46 IMOS National Reference Station Field Sampling • Preparation of the sample for flow cytometry of picoplankton • Samples are taken in triplicate. • Use a 1000µL pipette to add 1mL of the sample aliquot to a labelled 2 mL cryovial. • To the cryovial, then add 10 µL of glutaraldehyde (25%) whilst wearing protective gloves and eyewear. • Label according to the coding described in the sample coding section, above • Place these cryovials in a polycarbonate biological sample (e.g. urine specimen) jar to avoid potential contamination of other samples in the liquid nitrogen Dewar during storage. • Place the polycarbonate jar into the Dewar of liquid nitrogen for storage, or if from a remote locality, in the dry shipper for transport back to the lab with the HPLC samples and then placed into the storage Dewar. • The liquid nitrogen stored samples are long term stable, stored in a storage Dewar – maintained with liquid Nitrogen top-ups. These samples can then be shipped quarterly using dry-shipper Dewars and the supplied dry shipper freighting containers, to Hobart for analysis. NRS Sampling Manual Page 47 IMOS National Reference Station Field Sampling Protocol for IMOS/AMMBI microbial sample processing (new section June 2012) The following protocol was provided by Dion Frampton in May 2012. Equipment: • Inverter for 240V power to peristaltic pump; compatible with boat 12V power supply • Peristaltic pump (Watson Marlow 323S) • Pump head (313D2) with extension pump heads (313X2; total of at least 3 in parallel) • 3 or 4 x ~1m lengths of flexible tubing (compatible with cartridges) 2.4 mm outer diam.; one end with pre-filter fitting attached (using barbed end) – see next item • 3 or 4 x Pre-filter fittings – one end barbed, other end luer lock • Millipore Sterivex GP 0.22 µm filters (Cat. # SVGPL10RC) – one per sample • 6 x seawater containers (plastic; dark or covered ~4 L capacity) • 1 x Funnel (plastic; compatible with seawater containers) • 3 or 4 x 2 L plastic volumetric cyclinders • 50 mL centrifuge tubes or snap-lock bags; label with date/station/depth/volume filtered • Large eski to carry 4 L plastic containers (unless processing directly after sampling) • Small eski containing ice • Laboratory single-use gloves (e.g. latex) Sample processing : • As soon as practicable after sampling, set up equipment as follows (3 or 4 in parallel), making sure filter is kept in sterile pack until ready to use (after initial tubing rinse): Seawater containers >> pump >> Sterivex filter >> 2 L Vol. cylinders • Wearing gloves, place one end of tubing in seawater containers and pass tubing through pump cartridge into a volumetric cylinder (with barb/luer lock fitting attached, but not with filter attached). Run ~500 mL seawater through tubing (pump = ~300 rpm). • Attach Sterivex filter to fitting (luer lock), being careful not to touch lock end, and pass 2 L seawater through filter (as measured by cylinder which the filter should be emptying into). If filter blocks before 2 L has passed through, stop pump and record new volume. • After 2 L has passed through filter, remove tubing from seawater container and let pump continue for 1-2 mins (pumps remaining seawater through both tubing and filter). • Disconnect each filter and place in labeled centrifuge tube/snap-lock bag and then on ice in the dark (i.e. covered). Repeat steps 5 – 8 for next batch of sample depths. • Put individual samples into one larger snap-lock bag (labeled appropriately). Store all samples at -80 °C as soon as possible after processing. NRS Sampling Manual Page 48 IMOS National Reference Station Field Sampling Storage of all samples from a sampling trip At each NRS sampling, the samples taken are preserved or stored according to the detailed sampling methods laid out above for each sample type. In Summary: • Zooplankton: Two 500 mL jars of formalin preserved sample 1 cryovial of screened sample from the black jar for molecular analysis (in Dewar) • Phytoplankton, picoplankton and microbial studies: 1 litre of Lugol’s preserved sample stored upright, dark and sealed 3 cryovials of HPLC (pigment) samples. 1 for “water column” pigments and the other 2 for WQM pigment checks (in Dewar) 3 cryovials for flow cytometry (in Dewar) 6 labeled centrifuge tube/snap-lock bags for microbial studies from selected sites • Carbon samples: 1 per specified depth of square bottles for dissolved inorganic carbon 1 per specified depth of round bottles for Alkalinities • Hydrochemistry: 1 per depth of amber salinity bottles 3 per depth of polyethylene nutrient tubes (stored frozen upright) 2 filters for suspended matter stored in a cold dark place 1 filter for suspended matter (blank) stored in a cold dark place • CTD data: 1 profiling cast (checked on collection that the file contains data) • E-copies of log sheets: 1 per each sampling excursion of the combined field log sheet and the postcollection sample treatment NRS Sampling Manual Page 49 Handling liquid nitrogen to store samples prior to periodic shipments At remote sampling sites where it not possible to safely utilise a storage Dewar, or obtain liquid Nitrogen for regular top-ups, dry shipper Dewars can be used to freeze and transport samples that require freezing, to a main laboratory with a storage Dewar. Preparation of “dry shipper” Dewars is explained just below. At each NRS home base of operation there will be larger liquid nitrogen “storage” Dewars for those samples that have been described as requiring deep frozen storage for longevity and later transfer. Where there are “storage” Dewars, there needs to be a nearby bulk source of liquid nitrogen for regularly topping up the storage Dewars. It is necessary to have the ability to store samples for extended times to make allowance for the timing of sample transfer from the NRS to the laboratory for analysis – probably quarterly. Note: The Dewar must be stored in a well ventilated location to avoid potentially lifethreatening build up of levels of nitrogen as it turns to a gas. When transferring the cryovials or containers to or from liquid nitrogen, the wearing of protective gloves and safety glasses or face shield is essential. NRS Sampling Manual Page 50 IMOS National Reference Station Field Sampling Storage and shipping of all samples from a sampling trip Handling liquid nitrogen to transport samples in a dry shipping Dewar • Dry shipping Dewar preparation and upkeep • It should be noted that the liquid nitrogen preserved samples must be transported in a “dry shipper” using priority overnight freight as the freezing capacity of the dry shipper is limited • The dry shipping Dewar will need to be prepared about 3 days before it is needed. • Fill the Dewar with liquid nitrogen (the first time it will take quite a lot of liquid nitrogen because the Dewar is hot compared to the liquid nitrogen) then wait for 1 – 2 hours. After this time there should be no loose liquid nitrogen in the Dewar as it will have all been absorbed. • Re-fill the Dewar and wait 2-4 hours. Check the Dewar, probably all the liquid nitrogen will have been absorbed. • Fill the Dewar again and leave for 12 – 24 hours. After this time there will probably be some loose liquid nitrogen in the Dewar; this will indicate that the Dewar’s absorbent material is fully saturated. If the Dewar is dry repeat step 3. Return any loose liquid nitrogen to the storage Dewar that is kept in the laboratory. • The dry shipping liquid nitrogen Dewar will have a working time of about 10 days from when it is saturated with liquid nitrogen. For this reason it is possible to use them not only for shipping samples back to Hobart, but to also use them for freezing samples, in the interim, at remote sampling sites such as Esperance. • However if something goes wrong or you are delayed or you are over 10 days since saturating the Dewar get it filled with liquid nitrogen and then wait for at least an hour (longer is better). • If there is still loose liquid nitrogen in the Dewar it will have to be tipped out before the Dewar is taken to the airport. If the liquid nitrogen has been fully absorbed then fill the Dewar with liquid nitrogen again and wait for another 1-2 hours. • The Dewar must not travel with loose liquid nitrogen in it. • You will need to make sure the consignment note has “NOT RESTRICTED as per IATA SPECIAL PROVISION A152” written on it, otherwise the dry shipping Dewar is considered dangerous goods and the cost of transportation is 3-4 times more or it may be refused carriage. NRS Sampling Manual Page 51 Shipping samples preserved in liquid nitrogen Follow details of preparing the dry shipping Dewar (above) When the dry shipper is prepared as above, remove the following samples from the station storage Dewar and place in the dry shipper: • cryovials of screened sample from the black jar for zooplankton molecular analysis • cryovials of HPLC phytoplankton (pigment) WC samples • cryovials from the WQM depths for HPLC (pigment) analysis • cryovials for flow cytometry It’s very helpful if you pack each type of sample separately, held on cryo-canes or cryo-sleeves (plastic tubes) or tied in batches inside “knee-hi” stockings. Package and consignment note must carry the wording: “NOT RESTRICTED as per IATA SPECIAL PROVISION A152” Please dispatch the dry shipper, preferably by TOLL PRIORITY OVERNIGHT, or TNT Overnight or Australian Air Express (please do not use TOLL-IPEC Priority) to: Samples arriving Monday – Tuesday: Attention: Ros Watson CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Ph: (03) 62325268 or (03) 62325347 M: 0400567141 Email: ros.watson@csiro.au Samples arriving Wed – Friday: Attention: Lesley Clementson CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Ph: (03) 62325337 or (03) 62325347 M: 0409140230 lesley.clementson@csiro.au If you can’t reach one of the above, try other numbers and copy emails. • Remember, these liquid nitrogen stored samples should always be shipped in dry shipper Dewars – not on dry ice – as they will otherwise rapidly degrade. TSS samples should also be shipped to Ros Watson; for convenience they can be packed in batches and sent inside the travel case of the dry shipper (not frozen). Or they may be sent with the salinity crates as previously. NRS Sampling Manual Page 52 IMOS National Reference Station Field Sampling Storage and shipping of all samples from a sampling trip Transport of NRS samples to point of analyses: Unless noted elsewhere in the transport requirements, the samples can be transferred quarterly. Samples not preserved in liquid nitrogen Zooplankton: • Two 500 mL jars of formalin preserved sample per NRS sampling should be placed within the plastic sealed transport container provided. Ensure that the transport container is full (use empty jars, kitty litter, newspaper, etc as packing) and store upright • Dangerous goods transport requirements need to be checked and inserted if necessary re formalin • Because the formalin preserved samples are stable for some time they can be shipped to Dutton Park every 3 months (using TOLL). The street address is: Attention: Frank Coman CSIRO Marine and Atmospheric Research Ecosciences Precinct 41 Boggo Road Dutton Park QLD 4102 Ph: 07 3833 5917 Email: frank.coman@csiro.au Carbon samples: • After poisoning with mercuric chloride solution, the square bottles for dissolved inorganic carbon and the round bottles for Total Alkalinities are long term stable. • They are supplied in strong boxes (provided) for safe transport and should be shipped every 2 months to: Attention: Kate Berry CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7001 Phone: (03) 6232 5270 (W); (03) 6227 9589 (H) Email: kate.berry@csiro.au NRS Sampling Manual Page 53 Phytoplankton samples: • 1 litre bottles of Lugol’s preserved sample stored upright and sealed are long term stable when it is made sure to keep the PET sample bottles in a dark and cool spot, can be shipped in a sealed and dark leak proof container (perhaps every 3 months) to: For all sites except NRSMAI: Attention: Frank Coman CSIRO Marine and Atmospheric Research Ecosciences Precinct 41 Boggo Road Dutton Park QLD 4102 Ph: 07 3833 5917 M: 0419358195 Email: frank.coman@csiro.au For NRSMAI: Attention: David McLeod Australian Antarctic Division 203 Channel Highway Kingston TAS 7050 Ph: 6232 4325 M: 0438 443 658 Email: david.mcleod@csiro.au Suspended matter: filters should be stored in a cool dark place, but not frozen, and shipped (inside the dry shipper travel case is convenient) to: Attention: Ros Watson CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Ph: (03) 6232 5268 or 6232 5347 M: 0400567141 Email: ros.watson@csiro.au NRS Sampling Manual Page 54 Hydrochemistry samples: • Salinity - Amber glass salinity bottles are mid-term stable if stored in the dark in a cool position. Keep them in the sampling order inside the crates that were used when they were sampled. Ship to: Attention: Val Latham CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Phone: (03) 62325272 (W) or 0409325272. Email: val.latham@csiro.au • Nutrients – disposable polyethylene screw cap tubes (10 mL volume). Must be stored frozen, and shipped as soon as possible in a container with dry ice. Dangerous goods transport requirements for dry ice. You are required to use an agent or staff member who is trained in the packaging and transport of dry ice. The consignment note and the package must carry this description: UN1845, DRY ICE, and net weight of the dry ice e.g. “2 kg net”. The package must also carry a sticker for “miscellaneous dangerous goods.” Ship to: Attention: Val Latham CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Phone: (03) 62325272 (W) or 0409325272. Email: val.latham@csiro.au Microbial (molecular) genomics samples: • Filters must be stored at -80ºC until ready to send, and sent either monthly or quarterly, packed in dry ice and labelled as above. Attention: Lev Bodrossy: CSIRO Marine & Atmospheric Research Castray Esplanade Hobart TAS 7000 Phone: (03) 6232 5456 (W) or 0488405013 Email: lev.bodrossy@csiro.au NRS Sampling Manual Page 55 Uploading CTD datasets (under review): Advice from eMII June 2012 • Currently field samplers are uploading CTD files in .cnv format, which is the output from the Seabird processing software. • Files are named according to the BGC filenaming convention, with "CTDPRO" as the product name. These are the files that eMII have in public view. • Some field samplers have also uploaded raw binary (.hex) files, which eMII have archived. • This is going to change in the next few months. • eMII are working on adapting the IMOS Matlab Toolbox so that it can process CTD profile data and convert it to netCDF format. • Once that's in place, they will require CTD files to be uploaded in that format, though they will continue to archive the raw data. Details of the actual processing required (with Seabird software) are in the manual Tim Ingleton has written (the first link on this page: http://imos.org.au/anmndocuments.html). NRS Sampling Manual Page 56 IMOS National Reference Station Field Sampling ZOOPLANKTON ANALYSES Laboratory processing at CMAR Dutton Park Dry Weight Analysis: • The sample is drained of liquid by pouring it through a plastic plate with holes attached to an aspirator. A fine mesh (smaller than 100 µm) is placed over it to retain the sample. The sample is rinsed with distilled water • The sample is then scraped off the mesh (e.g. plastic knife) and placed on pre-weighed (to at least 3 decimal places) numbered pieces of aluminium foil. Numbering can be accomplished using the indentation left by a ball point pen without ink • The sample is dried (40-70ºC) over night (or for 24 hours) in an oven until dry • The aluminium dish and sample is then re-weighed and recorded Community Composition: • Analysis of the composition of the zooplankton community will be performed on the second formalin preserved sample. It is done using a dissecting microscope for easy to identify larger species and a compound microscope for identifying smaller difficult to identify species based on their appendages • Identification will be guided by the library of taxonomic keys we have assembled. Unknown specimens will be digitally photographed and sent for confirmation to expert collaborators • Copepods will be identified to species-level where possible • Other zooplankton groups will be identified to the highest taxonomic level possible • Quality control of the zooplankton identification will be maintained by annual taxonomic training with our national and international network of collaborators Zooplankton size spectra • This will be performed on the same sample as for the zooplankton community composition and subsequent to the microscopic analysis • We will separate the sample into 2 size classes before scanning (<1 mm and >1 mm) • We will scan the sample with our existing EPSON high performance scanner • We will analyse the scanned image using ZooImage software (customised in CSIRO by Nick Mortimer) Archiving The formalin-preserved samples for zooplankton community analysis will be archived in proponal phenoxitol at Dutton Park before microscopic analysis. Proponal phenoxitol is safer for using in the laboratory but formalin remains the initial preservative of choice for fixing zooplankton samples NRS Sampling Manual Page 57 Zooplankton Data Reporting/units: • • • Dry Weights (expressed as mg per m3) Community composition (expressed as species per m3) Average size (in µm) of the zooplankton community Are there alternate SI units to report these parameters in? NO NRS Sampling Manual Page 58 IMOS National Reference Station Field Sampling PHYTOPLANKTON ANALYSES Population Phytoplankton identification/cell counts NOTE: The Lugol’s preserved samples will be periodically transferred to Brisbane (from all sites except NRSMAI) or Hobart (NRSMAI) for analysis. • The samples will be transferred to 1 L measuring cylinders (volume recorded) and allowed to settle for at least 24 hours. • After this time approximately 900 mL will be siphoned off and the remaining sample will be transferred to a 100 mL measuring cylinder and again allowed to settle for at least 24 hours. • After this time approximately 90 mL will be siphoned off, the final volume recorded and thoroughly mixed before a 1 mL aliquot will be taken • The aliquot will be placed in a Sedgwick Rafter counting chamber and examined under an Olympus IX71 inverted microscope with phase contrast facility, DP70 camera and AnalySIS imaging software. • The counting method is based on Hötzel, G and Croome, R. (1998.). Precision of phytoplankton data (QA) • Staff from the same laboratory should conduct a ‘blind’ recount of the same sample once per sample trip. Recounts should yield precisions (density of top 10 species) that average ± 10% of initial count. Failure to meet this target indicates a need to review procedures. Accuracy of phytoplankton data (QC) • A minimum of 5% of samples should be split and analysed independently by a separate laboratory (and analyst). If accuracy is less than ± 20% for the estimated means of any of the top 10 taxa then methods and training should be reviewed. PLEASE NOTE Recipe for Lugol’s solution: (100 g potassium iodide, 50 g iodine, 1L distilled water, 100 mL glacial acetic acid.) Store Lugol’s solution in a dark ventilated container. NRS Sampling Manual Page 59 IMOS National Reference Station Field Sampling PHYTOPLANKTON ANALYSES Pigments HPLC pigments from the “water column” and for WQM Calibration. Phytoplankton pigments - Samples will be analysed by HPLC at CMAR with the established analytical procedure for pigment analysis using HPLC as follows: • All extraction procedures should be done under subdued lighting conditions. • Cut frozen filters into 3 or 4 pieces and place in a clean 10 mL centrifuge tube (wipe blades of scissors clean with a tissue between samples). • Add 3 mL of 100% acetone, cover tube with parafilm and vortex for ≈ 30 seconds. • Tubes are then placed in an ice-water/ultrasonic bath and the filter and acetone are sonicated for 15 minutes. • Store the tubes at 4°C for ≈ 18 hours or overnight. • Add 0.2 mL MilliQ water to each tube and sonicate in an ice-water bath for another 15 minutes. • Transfer filter and solvent quantitatively to a "Biorad" column containing a small GF/F filter acting as a plug. • The centrifuge tubes are rinsed with 2 x 0.5 mL 90:10 acetone:MilliQ water, which is quantitatively transferred to the respective "Biorad" columns. Each "Biorad" column is fitted into a clean 10 mL centrifuge tube and centrifuged for 5 minutes at 2500 rpm. • Record volume of extract in each centrifuge tube. • Wash "Anatop" filter with 1 mL of 100% acetone three times and dry filter by passing air from the syringe through filter (remove filter from syringe before drawing up air). • Take up about 0.5 mL extract from centrifuge tube in syringe, place filter on syringe and push 0.5 mL sample through filter to waste. Take up about 1.0 mL extract from centrifuge tube in a syringe. • Place filter on syringe and push 1.0 mL sample through filter into amber sample vial. NRS Sampling Manual Page 60 • • Only fill vial to 3/4 full. (Wipe tip of syringe on filter between extractand acetone wash). Repeat syringe and filter wash step in between samples. • Note: "Anatop" filters can be used for ≈ 25 samples. They should be washed as described above between samples and washed 3 times with acetone between batches. • Sample vials are then placed in the auto sampler holders for the HPLC analysis to take place. ** ensure this is still the current method ** NRS Sampling Manual Page 61 IMOS National Reference Station Field Sampling PHYTOPLANKTON ANALYSES Flow cytometry Flow cytometry analyses: Flow cytometry analysis for cells less than 3 µm were to be be performed at CMAR Hobart with a new Beckman – Coulter instrument however it was found that the selected instrument lacked the required detection capability. As of 2012, samples are analysed under contract at UWA using the method below. Picoplankton analysis by flow cytometry Photosynthetic picoplankton (Prochlorococcus, Synechococcus and picoeukaryotes) were enumerated by flow cytometry. Composite seawater samples from surface waters to a maximum depth of 50 m were collected from IMOS National Reference Stations every 1 to 3 months, depending on location. Subsamples of 1 ml were fixed in EM grade glutaraldehyde (0.25 % final concentration) for 15 min and quick frozen in liquid nitrogen until analysis (Marie et al. 1999). Samples were thawed at 37oC and 1 µm fluorescent beads (Molecular Probes) were added as an internal standard. Samples were analysed using a FACSCANTO II (Becton Dickinson) flow cytometer fitted with a 488 nm laser on high throughput mode at a flow rate of 60 µl min-1 for 2 min (Patten et al. 2011). Prochlorococcus, Synechococcus and picoeukaryotes were discriminated in scatter plots of red and orange autofluorescence of chlorophyll and the accessory pigment phycoerythrin (Marie et al. 1999). References: Marie D, Partensky F, Vaulot D, Brussard C (1999). Enumeration of phytoplankton, bacteria, and viruses in marine samples. In: Robinson JPEA (ed) Current protocols in cytometry, suppl 10. John Wiley & Sons, Inc, New York, pp 11.11.11–11.11.15 Patten, N.L., Wyatt, A.S.J., Lowe, R.J., Waite, A.M (2011). Uptake of picophytoplankton, bacterioplankton and virioplankton by a fringing coral reef community (Ningaloo Reef, Australia). Coral Reefs, 30:555–567 Units for Phytoplankton – population, pigments, flow cytometry • For optical phytoplankton study • Phytoplankton – counts will be expressed in cells per litre • Estimated phytoplankton biomass in mL L-1. • For HPLC pigments. • in µg.L-1 (or mg m-3) • For Flow Cytometry • to be notified Are there alternate SI units to report these parameters in? NO NRS Sampling Manual Page 62 IMOS National Reference Station Field Sampling DISSOLVED INORGANIC CARBON AND ALKALINITY ANALYSES Samples are returned to CSIRO Hobart for analyses using techniques developed for measurements in ocean waters on CO2/CLIVAR sections. The accuracy of the methods is checked against certified reference material from the Scripps Institution of Oceanography for each series of about twenty sample analysed. Detailed analytical procedures are provided in Dickson et al (2007). Carbon Parameters • Total dissolved inorganic carbon (TCO2), also known as DIC or CT Precision and accuracy estimate: ±1 μmol kg-1 • Total (titration) alkalinity (TALK) Precision and accuracy estimate: ±2 μmol kg-1 Total dissolved inorganic carbon: Total dissolved carbon dioxide in seawater is: TCO2 = [CO2]+[HCO3- ]+[CO3= ] Carbon dioxide dissolved in seawater is analysed by acidifying the seawater to convert bicarbonate and carbonate to CO2, extracting the CO2 from the solution by bubbling with high purity nitrogen (>99.995%), and trapping and quantifying the amount of CO2 using a UIC model 5011 coulometer. A SOMMA system is used to extract the CO2 and follows the procedure described in detail by Johnson et al (1993) and Dickson et al (2007). The SOMMA loads seawater from a sample bottle into a calibrated pipette thermostated at a constant temperature of 20°C. The sample in the pipette is then dispensed into a stripping chamber to which 1 mL of a 10% (v/v) solution of phosphoric acid has been added. The stripping chamber has a glass frit at the base and this is used to bubble nitrogen carrier gas through the sample and strip the CO2 from the sample. The CO2 in the carrier gas stream flows into the cathode compartment of a coulometer cell where it is quantitatively trapped in an ethanolamine solution. The absorbed CO2 reacts to form hydroxyethylcarbamic acid, causing a change in the colour of the cell solution due to the presence of a thymolphthalein pH indicator in the solution. Base is generated at the cell cathode, until the solution colour returns to its starting point. The efficiency of the coulometric method is determined by injecting known amounts of pure CO2 (>99.99%). Accuracy is checked by analyzing certified reference seawater from the Scripps Institution of Oceanography. NRS Sampling Manual Page 63 For each series of sample analyses, the general procedure is: • The coulometer cell is setup by adding UIC Coulometric Inc. solutions to the cathode and anode compartments, with the platinum cathode and silver anode connected to the coulometer. The gas stream from the SOMMA system is connected to the coulometer cell. • The power to the cathode and anode of the cell is switched on, followed by a series of injections of pure CO2 to condition the cell solution. The pure CO2 is added by switching an inline gas sampling valve with two loops of known volume (1.5 and 2.2.mL at 21.7°C). • Gas calibrations are next run to determine the efficiency of the cell. Values of between 99.5 ± 0.1% efficiency are considered suitable to begin sample analyses. Checks are also made to ensure there is a consistent blank and no evidence of leaks in the system. • A test seawater sample is analysed, followed by a certified reference material. If the certified reference material is within 2 µmol kg-1, the analysis of samples proceeds. • All samples are placed in a water bath (20°C) to ensure a constant temperature. The salinity of the samples is measured by the SOMMA system and used with the temperature of the sample to sample density Concentrations are in units of µmol kg1 . • Samples are analysed in batches of about 20 to 25 before a new cell and solution is required. • For quality control, two to three reference material analyses are made with each batch of samples. Total alkalinity method: An automated open-cell potentiometric titration is used to measure total alkalinity. The titrations are performed using a Metrohm automated burette to deliver acid titrant, and a combination Metrohm reference/glass pH electrode to track the progress of the titration. Sample volumes of 100mL are measured using a Metrohm dosino burette. The volumes delivered by the burettes are calibrated every six to twelve months by weighing volumes of deionised water dispensed by the burettes at 20° and applying an air buoyancy correction (Dickson et al 2007). The pH electrode responses are checked by comparison with Tris and Aminopyridine buffers in synthetic seawater (Dickson et al 2007). Electrodes with responses within 100 ± 0.3% of the Nernst slope of the electrode are used for titrations. The e.m.f. of the electrodes is recorded to ±0.1mV. The 0.1N HCl titrant contains 0.6 mol/kg sodium chloride to approximate the ionic strength of seawater. The normality of each batch of titrant is measured by coulometry and is known to better than ±0.03%. The density of the titrant, which is used to calculate the total alkalinity, is measured with an Anton Parr density meters over a range of temperatures near 20°C and is known to better than ±0.01%. NRS Sampling Manual Page 64 A non-linear fitting routine, written in IDL, is used to calculate TA. The routine is similar to the computation described in Johansson and Wedborg (1982) and Dickson et al. (2007). Comparison of the routine used here with a calculated TA result for data published in Dickson et al (2007) and using a different non-linear fitting procedure agree within ±0.01%. • Samples stored in sealed glass bottles are placed in a thermostated water bath and brought to a temperature of 20°C prior to analysis. • A 100mL volume of sample is pipetted into a water jacketed (20°C) glass beaker for analysis and the sample mixed with a stir bar. • A 0.1N solution of hydrochloric acid (HCl) titrant is added to the sample to adjust the pH of the seawater to about 3.5. The sample is then stirred for 10 minutes to degas CO2. • The titration proceeds by adding small increments of the hydrochloric acid titrant until the pH reaches about 3.0. The amounts of acid added and the associated change in e.m.f. of a pH electrode used to monitor the progress of the titration are recorded. About 20 data points are collected. • The total alkalinity is calculated using a non linear least squares technique. References Dickson, A. G., Sabine, C. L. and Christian, J. R. (2007) Guide for best practices in ocean CO2 measurements. PICES Special Publication 3, 191pp. Johansson, O. and Wedborg, M., (1982) On the evaluation of potentiometric titrations of seawater with hydrochloric acid, Oceanologica Acta 5:209–218 Johnson, K.M., Wills, K.D., Butler, D.B., Johnson, W.K. and Wong, C.S. (1993) Coulometric total carbon dioxide analysis for marine studies: maximizing the performance of an automated continuous gas extraction system and coulometric detector. Marine Chemistry 44: 167–187. NRS Sampling Manual Page 65 IMOS National Reference Station Field Sampling GENOMICS (MOLECULAR) ANALYSES All samples will be analysed at Hobart. Methods – Refer to Stanley Robert, Lev Bodrossy and Guy Abell capability yet to be fully developed. Methods for Genomic analysis • Zooplankton - To be developed • Microbial & phytoplankton – under development Units for Genomics (Molecular) results Genomics (Molecular) • under development – to be notified when initial results available Are there alternate SI units to report these parameters in? NO NRS Sampling Manual Page 66 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL HYDROCHEMISTRY ANALYSES Dissolved Oxygen (DOX) Like all the hydrochemistry analyses, temperature plays a significant role in effecting the results. The laboratory for dissolved oxygen analysis should be at a reasonably constant temperature. CMAR has employed a variety of methods to analyse for dissolved oxygen all based on a modified Carpenter technique. The first method was to manually titrate using a starch replacement (VITEX) visual endpoint on a sub-sample from a larger volume bottle. The next method employed (DODO) was an automated method based on an amperometric endpoint developed by Woods Hole Oceanographic Institute (Knapp et al, 1990). This again was analysis of a sub-sample. The current preferred method used by CMAR is based on a colourometric endpoint, developed by Scripps Institution of Oceanography. This method is a whole of sample bottle titration, is semi-automated and is driven by and results calculated by a M/S DOS based program. Currently, DOX analyses employed within CMAR is a mix of the above mentioned techniques. • The method, based on the SCRIPPS colourometric technique is currently in use only at sea on the National Facility – Southern Surveyor. • The Western Australia CMAR site uses the modified Woods Hole Oceanographic Institute method (potentiometric/electrode). • The Hobart site currently does not routinely analyse for dissolved oxygen NOTE: This is currently changing however, with the purchase of new equipment from SCRIPPS, in order to standardise the DOX analytical method utilised across CMAR (therefore IMOS NRS samples) and the National Facility. NRS Sampling Manual Page 67 Analysis using the SCRIPPS Dissolved Oxygen System Set up: • Check Dissolved Oxygen System water bath is clean and filled to approx. 1cm above window for light source. • If dirty, drain using drain tube on left-hand side of unit • Wash with milli-q • Wipe with Teri Wipe • Refill bath to 1cm above windows, ensuring no air is trapped on the source or detector windows • Turn on at power outlet: • 2 x Dosimats, 1 x stirrer, 1 x lamp, p.c. and printer (if required). • These may all run off a power board. • Ensure fan is unobstructed • Allow UV source approx. 15 minutes to stabilize • Mix thiosulphate in amber bottle in D.O. housing box. • Ensure thermocouples are in place on outside of bi-iodate and thiosulphate bottles and that liquid levels are above thermocouples • Open DOS window on p.c. • Change directory to bin. • Type in: O2 (Alpha-numeric). • Check date is correct. If so, press <Enter>. If not, change date. • Diagnostics are then carried out by the software. • The most common error is: “the Dosimat not ready” – usually improper seating of the Dosimat dispenser, or the Dosimat was started after the program was fired up. • Main menu will come up on p.c. display. NRS Sampling Manual Page 68 Check UV source by: • Check that the voltage of the detector (displayed above main menu on p.c.) is ranged between 2.7 – 2.8 volts • Adjust voltage using vernier on detector housing Flush burettes: • For bi-iodate solution flush manually using key pad • For thiosulphate, select “flush bottles” from the main menu • Sub-menu is displayed. • Place thiosulphate burette tip into small beaker. If the system has not been used for several hours, select “5 flushes” and press <Enter> • Whilst flushing, inspect lines of burette are free of air bubbles • The program indicates “not ready” whilst flushing is occurring • When flushing is completed, the software returns to the main menu Run Standards as follows: • After flushing, is complete, press <Enter> and type in Operator Initials • You will be prompted for record of reagent changes (new batches, etc.). Edit accordingly. • When finished, select “end input” and press <Enter> • Standards menu now displays default of “titrate standard”. • Select “Acquire bi-iodate Temperature” if this is the first standard to be titrated. Press <enter> to acquire temperature, then press <Enter> to return to the standards menu • Select “titrate standards” and press <Enter> • The software then prompts to verify the bi-iodate and thiosulphate temperatures. Then press <Enter> • Display is now in “ready” mode NRS Sampling Manual Page 69 • Add approx. 100mL of reagent grade water to flask, then add R3 (Sulphuric acid), R2 (Caustic/Iodide), R1 (Manganous salt) and then 10mL of bi-iodate from manual Dosimat. Mix well between additions. • Next add the spin bar. • Place the flask in the water bath in unit and ensure flask is seated flush to bottom and held in place by clamp. Put thiosulphate diffuser tip into flask – not in light path • Titrate by pressing <Enter> • The p.c.display shows the endpoint and slopes when the titration is complete. Press any key • Display now shows standards menu and is ready for next titration • Repeat titrations to 0.0003 (SCRIPPS use 0.0006) • When standards are finished, select “review results” and edit bad points as necessary • When editing is finished select OK and press <Enter>. • You can only edit 1 value at a time • Display is now on the standards menu • Select exit and press <Enter> • Display is now on main menu again Run Blanks as follows: • Select “Blanks” from main menu. Display now shows “Blanks menu” • Select “Titrate blanks” and press <Enter>. Thiosulphate temperature is displayed. If OK then press <Enter> and the “ready” screen will be displayed • Prepare blanks of reagent grade water ( approx. 100mL.) • • Add 1 mL each of R3, R2, R1, mixing well between adds. Then add 1mL of bi-iodate from the manual Dosimat • Press <Enter> and titration starts. • When endpoint and slopes are plotted, add a further 1mL of bi-iodate without removing flask from chamber. NRS Sampling Manual Page 70 • Press <Enter>. (repeat press <Enter> if titration has not started) • When second titration is finished press <Enter> • Repeat blanks until 3 or 4 are concordant to 0.0003 • Display is now on “Blanks” menu. Select “Review results” and press <Enter>. • Edit if necessary (only one edit at a time is possible) • Return to main menu by selecting “Exit” and press <Enter> • The system is now ready to run “Samples” Run Samples (unknowns) as follows: • Select “Samples” from main menu and press <Enter> • Display will show calibration (standard) data. If not, manually edit standardization data as follows: • Normality – leave as is, or edit if it has been changed. (e.g. change of bi-iodate standard) • Press <Enter> to enter values, or, if values are present and need changing, select “try again” at the bottom of the display. (e.g. thermocouple loose, wrong bi-iodate value in config. file, etc.) • Enter correct values (e.g. from previous session or if p.c. reboots) into appropriate fields on the p.c. from the printout. Note that the bi-iodate standardization information on the screen is not in the same order as on the printout. Press <Enter> • You will be prompted for a blank value. Enter the blank value and press <Enter> • Select OK and then press <Enter> • You will be prompted for Filename (cruise) and analyst (2 alphas). Enter information and press <Enter> • You will be prompted for oxygen case I.D. Enter information and then press <Enter> • Answer prompt for OK or try again and press <Enter> • The “Sample” menu will now be displayed and “Titrate” will be selected. Press <Enter> NRS Sampling Manual Page 71 You will be prompted to enter: • Station (CTD) number, then press <Enter> • Cast number, default to 1, then press <Enter> • Bottle number (Niskin #), then press <Enter> • number (sample flask #), then press <Enter> • Draw temperature (temperature of sample at sampling). Then press <Enter> • The “Ready” screen will now be displayed. • Prepare the sample by adding H2SO4 and spin bar. • Place the flask into holder, lower into bath ensuring that the flask is flat. Check that spin bar is operating properly. Ensure that the flocculent is fully dissolved. • Insert the thiosulphate diffuser into the flask. • The titration should now be in process. • At the conclusion of titration the display plots the endpoint and slopes. • Press <Enter> and the thiosulphate temperature is displayed. Press <Enter> • Two options will appear: Firstly • Next sample. If endpoints were OK accept this option by pressing <Enter>. • • Press <Enter> • “Comments” option will appear. Make comment if necessary, and then press <Enter>. • Fill in the next sample information as before, wait for “Ready” screen, then prepare sample and titrate. • Continue to end of samples. OR Over titrate. Choose this option if the slopes on the graphical display were not satisfactory, or if not satisfied with the spin bar action, outliers, etc. Only one over titration is allowed. Continue to end of samples. NRS Sampling Manual Page 72 Shut down procedure is as follows: • To shut down, select “End of titrations” from the main menu. • You are prompted to choose “OK” or “Try again”. • If OK, press <Enter> and return to DOS. • If leaving instrument for a period of time, shut down UV lamp at toggle switch on back of transformer. DOX Data handling is as follows: Deal with retrieving and transferring the DOX data as follows: • The filenames have no extension, so use DOS or XTGOLD to give the files an extension of .scp and transfer the files to floppy disk • When on the floppy disk, transfer the disk to the main hydrology p.c. Use HYDRO (dissolved Oxygen option) following the prompts and transfer the data. HYDRO expects files with the .SCP extension in order to read the data to file. • At the end of the voyage, make a subdirectory in the \bin directory, identifying your voyage with the format VYYCC. From the \bin directory, copy all the voyage titration files to the new voyage subdirectory. • The data can now be transferred into HYDRO – DISSOLVED OXYGEN options • “HYDRO” is in-house (CMAR) composed software package created by David Terhell to handle all data input, calculations and output. It is Windows compatible and is used throughout CMAR and aboard the National Facility for dealing with all routine hydrochemical parameters. • For more detailed information, refer to the SCRIPPS manual. NRS Sampling Manual Page 73 • In DOS type: dir/w <Enter> cd “SYYCC” <Enter> dir/w <Enter> move ..\”name of last run” . <Enter> move “name of last run” “name of last run”.scp (check this has been done by typing “dir/w” <Enter>) Insert a 3.5” floppy disk into “A” drive Type – copy “name of last run”.scp A: • The dissolved oxygen calculated is expressed as µML-1 Are there alternate SI units to report these parameters in? NRS Sampling Manual Page 74 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL HYDROCHEMISTRY ANALYSES Salinity analysis Before considering conducting any analyses using an instrument as sensitive to temperature change as the Guildline Salinometer, it is imperative to ensure that the room in which the instrument is housed is very tightly temperature controlled to less than +/- 2o C. All CMAR vessels and sites that routinely analyse salinity use the Guildline Autosal salinometer. Like any instrument that enables high-quality and high precision measurements for salinities; this instrument is calibrated each time it is used against an international seawater standard. The data obtained are measurements of conductivity ratio which are then converted using a formula, to practical salinity units (1978), or more correctly with no units e.g. a salinity value of 34.977. Because the samples when taken, will vary by a range in temperature, it is necessary to allow a period of time (say six to 12 hours) for the samples to equilibrate to the temperature of the analytical laboratory. Guildline provide an excellent manual with their instruments which details preparation, operation, maintenance, repairs to and circuit diagrams for the salinometer. It is not the scope of this user manual to replace the Guildline document but to provide sufficient operational detail for attainment of quality data. • Set up the salinometer according to the instructions provided in the Guildline manual. • As with any analysis, detailed personal instruction should be received from a qualified hydrochemist. • The salinometer should be in a controlled temperature room with the water bath temperature set at approximately 1 to 2° above the controlled laboratory temperature. • This is because the water bath is gently heated and the instrument cools passively. • Prior to commencing the analysis, turn the UPS or line filter on at the wall, then the instrument - the switch is located on the right-hand side at the back of the machine - and allow the water bath temperature to equilibrate for approximately 24 hours. • The samples and international standard seawater must sit alongside the instrument to overcome any temperature gradient in the room. If there is a temperature differential between sample and water bath, errors will occur. NRS Sampling Manual Page 75 Guildline salinometers in use within CMAR do not employ positive pressure subsampling as this method has led to accidents in the past. • Use of a peristaltic pump attached to the top of the salinometer has been found to be safer and effective. The peristaltic pump rate should not exceed 40 mL per minute because the pressure may break seals in the flow line. • The salinometers are also quite sensitive to electrical interference. Because of this they should be powered through a UPS or line filter and the tubing leading from the cell drain to a waste container must not touch the side of any wet surface. • Whilst the instrument and samples are settling to the ambient temperature of the laboratory, by looking through the small window check that the waterbath stirrer is operational and check that the heating elements (lights) are evenly oscillating on and off. • Also check that the analytical cell has been stored with pure water in it. • If the cell has been left dry it will need to be “wetted” with reagent grade water and let soak for approximately 6 hours. • To fill the cell: Turn the peristaltic pump speed control knob to the marked position which is equal to approximately 20 mL per minute (if the flow rate is too fast, turbulence and bubbles may be introduced into the cell.), and insert the polyethylene sample tubing into a beaker of reagent grade water. Stop the peristaltic pump and the cell will retain the reagent grade water. • Once the waterbath and samples have settled to ambient laboratory temperature, analysis can begin after the instrument is calibrated as follows. NRS Sampling Manual Page 76 To calibrate the salinometer take the following steps: • Place the silicon tubing for the peristaltic pump into position and clamp in place. • Turn the pump on but leave the flow rate at zero mL per minute. • Turn the salinometer pump on at the toggle switch on the front panel. • Place a finger over the flush valve and watch the analytical (conductivity) cell drain to waste. • Turn the peristaltic pump speed control knob to the marked position which is equal to approximately 20 mL per minute (if the flow rate is too fast, turbulence and bubbles may be introduced into the cell.), and insert the polyethylene sample tubing into a salinity standard left over from a prior analytical run. • Fill the conductivity sample cell to overflowing. Leaving the peristaltic pump running introduce a slug of air into the sample line, refill the cell to overflowing and re-flush the cell (by placing finger over the flush valve). • Repeat this until the cell has been flushed approximately 7 times. • Wrap a new unopened bottle of international standards seawater (IAPSO – International Association for the Physical Sciences of the Ocean) in thick soft paper towelling to prevent heat transfer from the hand to the solution. • Invert the bottle twice, break the metal seal, remove the rubber Grommet, wipe the sample intake tube with a tissue and insert the sampling intake tube midway down the standard bottle. • Flush the conductivity cell twice with the new standard. • Whilst the conductivity cell is filled with new standard stop the peristaltic pump control knob to zero mL per minute. • Turn the function knob from standby to the read setting. • Observe the LED display for approximately 5 seconds to get a stable reading. NRS Sampling Manual Page 77 • If the display flashes adjust the suppression knob until a stable positive reading is obtained. • If a stable positive reading cannot be obtained, make sure there are no bubbles in the conductivity cell; if so flush cell and refill. Note: do not flush cell in the read mode. • Whenever the cell is filled, gently wipe the sample Inlet tube with a tissue. • Refill the conductivity cell with clean standard seawater. Turn the function knob from standby to read and mentally note the average number of the LED display – conductivity ratio reading - (stable to +/- 0.00002). • Turn the function knob to standby, flush the conductivity cell and refill, and take another reading from the conductivity ratio display. • Repeat the measurement procedure until the conductivity ratio display, delivers two consecutive positive readings within +/- 0.00002. • The conductivity ratio reading should be exactly twice the K15 value on the seawater standard label. • If this is not the case, release the lock on the standardise vernier knob and turn the knob until the conductivity ratio display is reading twice the K15 value. • Re-lock the standardise vernier knob when the conductivity ratio display is correct. • If the standardise knob has had to be changed, check the reading of the vernier against the record of the standardise setting from the previous calibration at that bath temperature (instrument logbook). • There should be close agreement between the previous reading and the current reading. • If the standardise value has varied by more than 1.00, at that bath temperature, there may be a faulty thermistor; standard may be faulty; the analytical technique may not have been proper, a different bath temperature may have been used or the cell may not have been properly filled. NRS Sampling Manual Page 78 • Again test the IAPSO standard seawater, open a second bottle and go through the standardisation procedure again. When you are satisfied that machine has been properly standardised, turn the function knob to zero (this figure will indicate any possible instrument drift - check during the duration of analytical run). • Record the following readings: standby, zero, and the standard (seawater standard conductivity ratio), as well as the date, analyst, lab temperature, water bath temperature and the instrument number. • These should all be clearly laid out on the analysis sheet as well as in the salinometer logbook. • Standby value will change if there is sufficient change in the laboratory temperature or the standardise value has been accidentally altered. • In controlled operating conditions the standby value should not change. • If it has changed by more than 1 unit it is necessary to re-calibrate the instrument. Salinity Sample (unknowns) measurement. • Remember that the sample must have equilibrated at least six hours to a constant laboratory temperature, that the standardise knob must not be touched during analysis of “unknowns”, the analytical cell must be bubble free and that the cell must never be empty whilst the function knob is in the read position. Taking sample measurements is a similar procedure to calibrating the instrument. All the same precautions and meticulous attention to detail must be followed in order to obtain reliable accurate readings. Further details about taking readings of samples follow below. • Fill the bottle numbers onto the analytical sheet in the order they were sampled. • Turn the Guildline pump toggle switch to the on position and set the peristaltic pump to the sample speed. • Place finger over flush valve to pump cell contents to waste. • Gently invert the first sample bottle two or three times to ensure thorough mixing. • Hold the sample bottle in a thick soft paper towel to avoid heat transfer from hand to sample. NRS Sampling Manual Page 79 • Fill and flush the cell two or three times. • Whilst the cell is full turn the function knob from standby to read and observe the (LED) conductivity ratio display. If it is flashing adjust the suppression knob until a stable positive reading is obtained. • Flush and fill the cell (remembering to keep the function knob on standby when the cell is empty) and take readings of the conductivity ratio using the function knob until two consecutive measurements agree to +/- 0.00002 conductivity units. • Record this value on the analytical log sheet ensuring the value is recorded against the appropriate sample bottle number. • Remove the sample intake tube from the bottle, wipe the tube, cap the bottle with the residual sample in it, turn the peristaltic pump off and return the sample bottle to the crate. • Repeat until all samples are finished and recorded on the analytical sheet. • When the crate of samples is completed, using the function knob check and record the standby and zero readings. • Should the original standby value have drifted more than +/-1 unit, unseal another international seawater standard and treat it as another unknown sample. • Repeat the readings as you would any other unknown sample and record the value of the conductivity ratio. • If the instrument has drifted only very slightly this reading can be used to correct for instrumental drift and therefore attain corrected values of the unknown samples. • Any major drift indicates a problem either with the instrument, the temperature control of the laboratory or the operator. If any major drift is instrumental, refer to the Guildline manual for trouble shooting. NRS Sampling Manual Page 80 Salinometer shut down procedure • Clean up any stray seawater with a wet cloth. • Using a beaker of reagent grade water draw water up and into the cell using the peristaltic pump as well as the flush valve until approximately 10 or 12 thorough rinses of the analytical cell has been achieved. • Leave the conductivity cell filled (with no air spaces) with reagent grade water. • Turn the toggle switch for the Guildline pumps to the off position. • Turn the peristaltic pump off and remove the silicon tubing from the pump head. • Turn the Guildline off at the switch at the rear of the instrument. • Turn the UPS or line filter off at the wall. • Update the logbook for the salinometer with all the settings used for that run. • From the data obtained from the Guildline and the software provided, calculate and record the salinity of the unknowns. Salinity Result reporting • Used to be expressed as p.p.t. or psu and determined on the practical salinity scale however it is now generally accepted by oceanographers as a number without a unit expression. * e.g. 34.432 * McDougall, T; verbal communication NRS Sampling Manual Page 81 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Suspended Matter (SM) Analysis (note: check with Ros) Filter preparation: • Filters for TSM analysis are prepared in the following manner prior to field sampling. • Place individual 47 mm GF/F filters on a sheet of aluminium foil and cover with another sheet of foil. • Place in muffle furnace and set temperature to 450o C. • Once the furnace has reached 450o C, leave it at this temperature for approximately 1 hour and then turn the furnace off. • When furnace is cool remove filters. • Rinse filters in Milli-Q water for 1 hour then remove each filter from the water using forceps and place on a clean numbered glass petri dish which contains 3 small balls of aluminium foil. • Place petri dishes on a tray (a shallow cake tin is ideal) cover with a sheet of aluminium foil and place in an oven at 75C for approximately 3 hours. • Remove from oven and let cool for around 15 minutes. • Weigh each filter, record weight on sheet and return to the same petri dish. • Return petri dishes to the oven at 75o C for approximately another 2 hours. • Remove, cool and weigh again. • Generally after 2 weighings, the filters should have reached constant weight. If there is more than 0.2 mg difference between the first and second weighing, repeat the drying/weighing process. NRS Sampling Manual Page 82 • Once the filters have reached constant weight store in the appropriately numbered Millipore Petri-slides until required. On TSM log sheet record the number of the Petri-slide along with the weight of the filter stored in the Petri-slide. • Always do the initial and final post-sampling weighing of the filters on the same balance. Suspended Matter (SM) Analysis (total, organic and inorganic) • After the NRS Samplers have collected the Suspended Matter sample in the field: • Place filters in glass petri dishes, each labelled with the same number as that on the petri slide from which each filter came. Each petri dish will contain 3 small balls of aluminium foil on which the filter will sit • Place petri dishes on a tray (cake tin), cover with a sheet of aluminium foil and place in an oven at 75o C for approximately 3 hours. • Remove from oven and let cool (around 15 minutes). • Weigh each filter, record weight on the TSM log sheet against the same number and return the filter to the same petri dish. • Return petri dishes to the oven at 75o C for approximately another 1-2 hours. • Remove, cool and weigh again. • Generally after 2 weighings, the filters should have reached constant weight. If there is more than 0.2 mg difference between the first and second weighing, repeat the drying/weighing process. • Determine the TSM weight by subtraction of the pre- filtration weight from the post-filtration weight. • Take note of the sample volume that was filtered through the filter. • Calculate the weight per volume (Total). • Return filters to glass petri dishes and place petri dishes on the floor of a muffle furnace (note the position of each of the numbered dishes as the numbering on the dishes will be removed during the muffling process). Cover the dishes loosely with a sheet of aluminium foil and program the muffle furnace to 450o C. After the furnace has reached this temperature, wait 3 hours before programming the temperature of the furnace to 20o C. When the furnace has reached 20o C, remove the dishes and filters and weigh immediately. . NRS Sampling Manual Page 83 • Determine the weight of the inorganic fraction by subtraction of the pre- filtration weight from the post-filtration muffled weight. Calculate the weight per volume. • Determine the weight of the organic fraction by subtraction of the inorganic fraction weight from the total TSM weight. Calculate the weight per volume • This analytical procedure is also followed for the “seawater blank” that was carried out at the time the suspended solid sample for the same station was filtered. • As mentioned in the sample filtration procedure there is a need to have a “ blank” filter for comparison to the actual sample filters. The procedure for filtering the blanks is described in detail in the sample treatment section. Basically it is just necessary to prepare and send off an extra filter in a petri dish for each station to use as a blank at each sampling. NRS Sampling Manual Page 84 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL HYDROCHEMISTRY ANALYSES NUTRIENT ANALYSES CMAR used to use segmented flow analysis for nutrients, however, flow injection analysis (FIA) is now used as the method of choice both at sea and on land. Both methods use the principle of colourometric analysis for a number of analytes simultaneously. For oceanographic purposes the FIA instruments (LachatTM) are routinely used for the analysis of dissolved inorganic phosphate, nitrate plus nitrite and reactive silicate. Lately work is being done to develop a method to enable analysis of trace levels of ammonia using a fluorescence detection method. The FIA instruments were selected to replace the segmented flow instruments for faster throughput of samples, the need for a lower sample volume and the ability of the Lachat software to deal with the difference between salt water and freshwater matrices in a far simpler way. The FIA system is designed and software driven in such a way as to minimise operator error or variance. The variable speed proportioning pump with the array of tubing of various inner diameters carrying the wash solution, sample solution, and reagents for each chemistry, is still the heart of these automated systems. The sample and wash solutions are mediated by an XY sampler. The sample stream is split into the desired number of chemistries, with the relevant reagents being added during transit to that colorimeter with an interference filter appropriate to that chemistry. For calibration purposes, calibrants of known concentration are introduced into the carrier stream into which are introduced the reagents. The resultant degree of colour which develops being proportional to the known value of each calibrant. The degree of colour which develops in unknowns is compared to the degree of colour interpolated from the known calibrant values; corrections are made using the software and the value of the unknown is calculated. NRS Sampling Manual Page 85 Operation of the Instrument • Like any automated/semi-automated analytical system, the same steps and procedures for setup as well as shut down and operation should be followed to ensure consistency, accuracy and minimisation of variability between operators and instruments. • Turn the computer on, open “OMNION” the Lachat. • Initialise the auto sampler, prime the dilutor and start the pump • Change the pump tubes to reagents and ensure all lines are flushing correctly • Set up the method for the run (using OMNION), check the timing and prepare a new cadmium column • The calibrants are prepared fresh each run by using the Lachat dilutor and the stocks should be at the laboratory temperature. • Ensure that the calibrants chosen cover the range that may be anticipated for the unknown samples. • Making up the mixed calibrants (and reagents) is covered elsewhere. The usual ranges of calibrants however, for blue water oceanography, are as follows: • Silicate: 0, 7, 14, 21, 28, 35 or 0, 28, 56, 84, 112, 140 (uM per litre) • Nitrate (+Nitrite): 0, 7, 14, 21, 28, 35 (uM per litre) • Phosphate: 0, 0.6, 1.2, 1.8, 2.4, 3.0 (uM per litre) • Nitrite: (TBD) • Ammonia: (TBD) – Analytical capability being refined in Hobart • Like all colourometric methods it is necessary to determine Method detection limits and precisions prior to commencing analysis for unknowns. This involves running a number of blanks, prepared standard reference materials, a number of "unknowns" (say 15) samples from the one Niskin bottle, a QC sample and nitrite to check the efficiency of the cadmium reduction column. NRS Sampling Manual TM (the Lachat software) and turn on Page 86 • At the start of the day ensure all solutions and frozen samples reach ambient laboratory temperature. • Prepare fresh reagents for the analytes that are to be tested for the day as well as fresh standards and intermediate standards. • Flush the system for 15 minutes with carrier solution water and reagents ensure that the cadmium column is not switched in, • The carrier solution is matched as closely as possible to the sample salinity – i.e. Artificial Sea Water (ASW)or pure reagent grade water (PW) • Use this time to complete preparation of fresh reagents. • After 2 minutes turn the cadmium column in to refresh the buffer, or make a fresh cadmium column. • The chemistries which are used by the Hobart hydrochemistry group, are methods developed by Lachat and run directly as outlined in the following list: QuickChem Method 31-115-01-1-I Determination of Orthophosphate by Flow Injection Analysis QuickChem Method 31-107-04-1-A Determination of Nitrate in brackish or seawater by Flow Injection Analysis QuickChem Method 31-114-27-1-D Determination of Silicate by Flow Injection Analysis QuickChem Method 31-107-06-4-A Determination of Ammonia in Brackish or Seawater by flow injection analysis (To be increased in sensitivity by further CMAR modifications) • Once the system is stable – usually only 15 minutes or so, the run can be started. • At the end of the day/run the system is flushed for about 15 minutes on reagent grade water, and then shut down. NRS Sampling Manual Page 87 Reporting Units for Nutrients o µM-1 Nitrate/Nitrite o µM-1 Silicate o µM-1 Orthophsphate o µM-1 Nitrite o µM-1 Ammonia (when capability fully developed at Hobart) Dissolved Oxygen (chemically determined) • Following further consideration this will only be carried out for 2 National Reference Stations • Reporting units for dissolved oxygen • μmol/L For Suspended Matter (Total, Organic and Inorganic) • as mg L -1 Salinity reporting • It is now generally accepted by oceanographers as a number without a unit expression. e.g. 34.432, as described at the conclusion of the salinity analysis section. Are there alternate SI units to report these parameters in? A Summary Table of Reporting Units for all Analysed Parameters will be inserted in Here later. SI units MUST be used wherever possible. NRS Sampling Manual Page 88 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Data handling, Archival and Retrieval Field and sampling data for National Reference Stations • To overcome a broad range of different data processing and entry methodologies utilized by the broad range of participants in this National study; the processing of the data will be carried out by CMAR at the Dutton Park and Hobart sites. • These need to be assessed and the most efficient and comprehensive means of data processing and entry for each parameter should be utilized rather than using “hybridised” means. It also eliminates the inter laboratory cross checks and inter comparisons that would be necessary to ensure consistency • After each site has been sampled, analysed and processed the data will be entered – and checked for correctness. • The data will be entered only on a dedicated p.c. that is regularly backed up onto the network • For all field log sheets, filtration logs, notations, all CTD data files, etc., please keep the original “hard” copies • Upload to https://df.arcs.org.au/ARCS/projects/IMOS/staging/ANMN all the above information whether “raw”, “processed”, documents or log sheets in printed PDFformat, i.e. not hand written PDF scans. Uploads should be contained within a suitably titled folder. Instructions for obtaining ARCS access are included at the end of this document. • Any non-standard data loading tools for loading the information into the eMII database will be developed by UTAS eMII project staff. • When the data has been captured in electronic format and each lot of data (both raw and corrected) has been uploaded to the database staging directory as above, contact staff at eMII and advise that this has been carried out. NRS Sampling Manual Page 89 Analytical results (including QA/QC) • As soon as the analytical results for the sampling sites become available the data should be checked for appropriate QA/QC, entered – and checked for correctness. • The data will be entered only on a dedicated p.c. that is regularly backed up onto the network • The analytical log sheets, analytical results, notations, CTD data files, etc., will be copied in electronic format, as with the field data, to the ARCS hosted staging area https://df.arcs.org.au/ARCS/projects/IMOS/staging/ANMN • When the data has been captured in electronic format, each lot of data will be copied to the UTAS eMII database (both raw and corrected), and the contact staff at eMII advised that this has been carried out. Archival of raw data sheets • When field log sheets, post-collection sample treatment log sheets, analytical sheets, notes of special comment, result sheets, etc. are brought in or completed, they need to be stored in an e-printed form. Printing vastly improves the legibility of the data for the end user.The form records need to be stored within a dedicated p.c. that is regularly backed up on the network. • After the hardcopy field-sheets are converted into electronic form (or vice versa), the hard copies will then be stored in a secure local location • For transfer, the e-forms should be converted to PDF. Each lot of data will be uploaded to the ARCS hosted staging area: https://df.arcs.org.au/ARCS/projects/IMOS/staging/ANMN (both raw and corrected), and the contact staff at eMII will be advised that this has been carried out. It is important to stipulate exactly what files have been uploaded and by whom. NRS Sampling Manual Page 90 Access to and retrieval of all data acquired • Data is initially forwarded to an ARCS hosted staging area. From there it can be accessed by analytical teams if required. The data can then be made publically available by eMII via the IMOS data portal. • Biogeochemical data collected at the IMOS National Reference Stations is required to be freely accessible to all participants in a uniformly formatted output file(s). • The electronic data should be accessed from a central repository accessible by all: the UTAS eMII project • Data used from IMOS collections for reporting or other reasons should acknowledge IMOS and the I.P. acknowledged to IMOS and the Lead Scientist for a Data Group/Type as laid out in the Project Charter. Please note that as a matter of protocol all files – raw, processed, log sheets, or any recorded form of information that should be in a digital format – that are to be passed between the NRS samplers, analysts, processors and the eMII should be transmitted as station inclusive, non-password locked *.zip files. This avoids file entrapment by virus scanners in email servers. Data Management and the Lead Scientist • The Lead Scientist, as identified in the IMOS National Reference Project Charter, should be responsible for ensuring that the data from the various analytical staff have been compiled, backed up on the network and copied (raw and processed) into the eMII database. • The Lead Scientist should conduct some quality control by reviewing the incoming data on a regular basis searching for possible anomalies. • Possible anomalies should be re-checked and, if necessary, the data corrected. *The Lead Scientist should be reasonably familiar with expected values for each parameter at each station during each sample period. Determination of “The Lead Scientist” has been explained in the project charter and was determined by responsibility according to “data type”. NRS Sampling Manual Page 91 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL References World Ocean Circulation Experiment – Operations Manual, Volume3; WHP Office Report WHPO 91-1 – WOCE Report No. 68/91, Revision1. To be added to CSIRO Marine Laboratories Report 236, 1999. Rebecca Cowley, Gary Critchley, Ruth Eriksen, Val Latham, Ron Plaschke, Mark Rayner and David Terhell. Bucklin A (2000) Methods for population genetic analysis of zooplankton. In Zooplankton Methodology Manual. Edited by Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M. pp. 533-570 Heron AC (1982) A vertical free fall plankton net with no mouth obstructions. Limnology & Oceanography: 380-383 Hötzel, G and Croome, R. (1998.) A Phytoplankton Methods Manual for Australian Rivers. Occasional Paper 18/98, Land and Water Resources Research and Development Corporation, Canberra. 52pp NRS Sampling Manual Page 92 IMOS –Biogeochemical Sampling– field log sheet UTC Date: UTC StartTime Wind Speed: Latitude: / / Personnel Sonic. depth: Wind Dir: Longitude: Site Code: Cloud Cover: Tide: Vessel: /8 Calculated Secchi Disc Depth Cast Depth: Start Time: File Name NISKIN BOTTLES- FIRST CAST Depth: Surface ALK DIC Niskin #: Air Temp: Start/Finish Time Salinity # / DOX# Carboy Vol. DOX# Carboy Vol. DOX# Carboy Vol. DOX# Carboy Vol. DOX# Carboy Vol. DOX# Carboy Vol. Nutrients # ALK Depth: DIC Salinity # Nutrients # Niskin #: ALK Depth: DIC Salinity # Nutrients # Niskin #: ALK Depth: DIC Salinity # Nutrients # Niskin #: Depth: Niskin #: ALK Depth: ALK DIC Salinity # Nutrients # DIC Salinity # Nutrients # Niskin #: NISKIN BOTTLES – SECOND CAST Start/Finish Time / NISKIN BOTTLE- CAST FOR WQM PIGMENT CALIBRATION Depth: ALK DIC ALK DIC ALK DIC ALK DIC DOX# Carboy Vol. Salinity # DOX# Carboy Vol. Salinity # DOX# Carboy Vol. DOX# Carboy Vol. Nutrients # Niskin # Depth: Salinity # Nutrients # Niskin #: Depth: Carboy Vol. Nutrients # Niskin #: Depth: DOX# Nutrients # Niskin #: Depth: Salinity # ALK DIC Salinity # Nutrients # Niskin #: Upper Depth Niskin # Time Collected Lower Depth Niskin # Time Collected NRS Sampling Manual Page 93 IMOS NRS Biogeochemical post sampling TSS filtering (“Water column”) - Carboy UTC Date UTC Start time Site Code Filter No. Vol. Filt. (L) Comments HPLC pigment filtering (“water column”) – carboy - aim for 4 litres Start time Vol. Filt. (L) Comments HPLC pigment filtering (5 litre sample for UPPER WQM) –aim for 4 litres Start time Vol. Filt. (L) Comments HPLC pigment filtering (5 litre sample for LOWER WQM) –aim for 4 litres Start time Vol. Filt. (L) Comments Microscopic phytoplankton -Species composition ( “Water column” Carboy) Start time Sample preserved Y/N Comments Flow cytometry - (“Water column” Carboy) Start time Sample preserved Y/N Comments Zooplankton Genetic filtering (“Black Plastic Jar”) – Drop net Start time Conc. Sample In Cryovial Cryovial in Dewar Comments COMMENTS NRS Sampling Manual Page 94 IMOS National Reference Station Biogeochemical Sampling Labeling of sample containers Zooplankton:Each sample bottle should be labelled both inside on waterproof labels written on in pencil and also have an outside label reflecting the contents of the jar, as noted on the internal label. See examples below. IMOS NRS ZOOPLANKTON – PRESERVED in FORMALIN Date time Site Code replicate number comments IMOS NRS ZOOPLANKTON - UNPRESERVED Date time Site Code replicate number comments Carbon: will come in pre-labeled bottles and details noted on log sheets Suspended Matter: will come in labeled Petri dishes Lugol’s’ preserved phytoplankton: should be clearly labeled and recorded on log sheet Salinity: bottles will be clearly visible on the bottle and records recorded in the log sheet Nutrients: the tubes should be clearly labeled prior to sampling and freezing Cryotubes: must be written on with a special cryopen concisely describing the contents of the tube NRS Sampling Manual Page 95 IMOS National Reference Station Field Sampling BIOGEOCHEMICAL OPERATIONS MANUAL Listed below, is a summary of sampling equipment and storage items to take on the vessel for a sampling trip. Work Type Item Type - Description Check Box Vessel to include GPS 4 or 5mm. Non-conducting cable winch for overside package retrievals davit 25 kg. hydro-wire weight deck hose with spray nozzle cray pot hauler or similar Miscellaneous field log sheets pencils PFD's MSDS for formalin and mercuric chloride Water Clarity secchi disc secchi rope - marked in 1m intervals Shackle to attach rope eye splice to disk CTD CTD with sensor array for Temp., Pressure, Cond., Fluorescence, Turbidity + cage Laptop Zooplankton Zooplankton drop net drop net cod-ends 12mm "Silver rope" fro drop net of a length appropriate for the depth of the sampling station Fish box to contain "Silver rope" plastic 500mL jars Black jars labels for 500mL jars stopwatch formalin Formalin dispenser with cap Container to hold Formalin dispenser Niskin Sampling NISKIN GEAR NRS Sampling Manual 5 litre niskin bottles bronze messengers Page 96 DISSOLVED OXYGEN (DOX) Dissolved Oxygen (DOX) sample bottles Reagents for DOX preservation Reagent dispensers for DOX reagents CARBON Tote boxes with DIC and ALK bottles Plastic box with mercuric chloride bottle Plastic box with pipette and sampling tube SALINITY Salinity bottles in transport crates NUTRIENTS INTEGRATED SAMPLE Icepack for nutrient samples Eski/polystyrene container triplicate labelled nutrient tubes in rack Large mouthed 3 litre graduated jug for measuring niskin excess 20 litre natural plastic carboy Large plastic funnels shadecloth WQM SAMPLES NRS Sampling Manual 2 X 5 litre carboy - natural plastic Page 97 IMOS NRS BIOGEOCHEMICAL SAMPLING WORKFLOW Vessel at sea to collect NRS biogeochemical information Fill in station log and all sample logs fully and comprehensively Conduct and record Secchi disc reading With sensors for: Temperature, Pressure, Conductivity, Fluorescence, Turbidity, GPS, Time, Date, Salinity (derived) and Dissolved Oxygen Conduct CTD profile Conduct 3 zooplankton drop nets Preserve one sample Preserve second sample Download RAW and PROCESSED CTD data. Upload to IMOS staging directory Place third sample in jar un-preserved Conduct water sampling, using 5 litre Niskins on a wire cast; with bottles at surface, and then 10 m intervals to 50m (where possible). Take 2 x 5 litre bottles at the depth of each WQM - on the mooring for that NRS site - and some other “selected” depths. The “extra” niskin bottles sampled at WQM depths is for purposes of comparison to/calibration of, the sensors on the WQM’s. Sample for microbial genomics from nominated depths Dissolved Oxygen at 2 stations From each niskin bottle, sample for biogeochemical samples shown below Carbon & Alkalinity Salinity Take samples & carboys to the shore base for any further sample preservation / preparation. Keep cool and dark. Filter for HPLC Pigments Store at -80oC. Sampling for Microbial Genomics under development Sample for Flow cytometry Store these samples in liquid Nitrogen in a shore based Dewar NRS Sampling Manual Sample WQM bottles for pigments Nutrients frozen on dry ice Cover, keep cool and dark Filter for total Suspended solids. At analysis, will test for total, organic and inorganic Measure & combine residual waters, based on the surface residual, in to a carboy Filter for HPLC Screen the “live” sample and scrape off residue into a cryo-vial Preserve sample with Lugol’s Solution for Phytoplankton microscopy Store in the dark and cool (fridge) Page 98 IMOS NRS BIOGEOCHEMICAL SAMPLE TRANSPORT AND ANALYSIS Transport each 3 or so months Transport directly to CMAR Hobart Laboratory Dissolved Oxygen from selected sites Nutrients in a container of dry ice in for transport overnight Microbial genomics from selected sites, on dry ice for transport overnight 1 Cryovial for HPLC. 2 Cryovials for WQM HPLC. 3 Cryovials for flow cytometry work. 1 or more Cryovial for Zooplankton genomics Analysed within 48 hours of collection by hydroche mistry Transfer to freezer until analysis by hydroche mistry Transport the following samples in the supplied tote boxes to analytical labs (CMAR) Transport the following samples in the “dry” shipper Dewar to analytical labs (CMAR) Hobart Transfer vials for zooplankton & microbial genomics to a -80oC freezer Alkalinity, DIC samples and salinity samples (Hobart) Transfer cryovials for HPLC and Flow Cytometry to lab, store in Dewar of liquid nitrogen Store in Carbon Lab until samples can be analysed by Carbon Group Suspended matter filterskept cool (Hobart) Transfer to fridge until analysis by hydroche mistry Samples preserved with Lugol’s (Dutton Pk, except NRSMAI) Samples preserved with Formalin (Dutton Park) Store upright in fume cupboard until analysis Carry out analyses (as capabilities permit) of all parameters; store the raw and processed data on a secure computer that is regularly backed up. Copy all field sheets and laboratory log sheets, notes, etc to .PDF format, keep hard copies and upload electronic copies to: IMOS staging directory NRS Sampling Manual Inform UTas when uploads are done Inform eMII when uploads are done Copy all raw - and processed analytical data - and upload electronic copies to: IMOS staging directory Store upright in fume cupboard until analysis All log sheets, methods, manuals, reports, versions, etc. stored in the UTAS IMOS data base are freely available for all Page 99 ARCS REGISTRATION for DATA UPLOADING 1. Register for an ARCS IdP: The ARCS Identity Provider (ARCS IdP) is open to anyone in the Research & Education community within Australian and New Zealand as well as to legitimate international researchers needing access to eResearch services and resources in Australia and New Zealand. It is primarily intended for use by individuals who do not otherwise have an IdP elsewhere. Go to https://idp.arcs.org.au/idp_reg/ NRS Sampling Manual Page 100 2. Receive an email from ARCS to confirm receipt of your application no-reply@arcs.org.au to me show details 10:52 AM (0 minutes ago) Hi Kate, Thanks for applying for an ARCS IdP identity. To confirm your email please visit https://idp.arcs.org.au/idp_reg/requests/confirm/ and enter the confirmation code shown below: WluPAco3XpROYuO3g072 Once you have confirmed your email address we will process your account request. You will receive an email when your account has been approved. Thanks, ARCS IdP Administrator 2. Await email approval of your IdP from ARCS. 3. Use your new IdP to register for ARCS services at http://access.arcs.org.au 4. Receive an email approving use of ARCS services from ARCS. 5. Login to the ARCS Data Fabric for the first time at http://www.arcs.org.au/index.php/arcsdata-fabric 7. Email Marty Hidas ( Marty.hidas@utas.edu.au) for access to the IMOS data directories NRS Sampling Manual Page 101