My Sandbox lab Introduction: Everyday, wind and water pick
advertisement

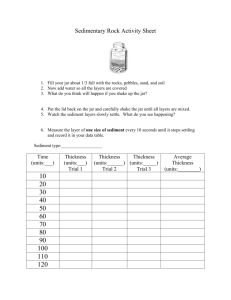
My Sandbox lab Introduction: Everyday, wind and water pick-up and carry and deposit sediments on Earth’s surface. The sediments form layers based on two important properties; particle size and density. In this experiment, you will measure both properties and see how they affect the layers of sediment that form in a jar of water. Which property is more important? Will the densest or the largest-sized particles end up on the bottom of a jar of sediments shaken in water? Prediction: Materials: sand, beaker, hand lens, 4-5 square pieces of aluminum foil, bucket or plastic shoe box, 1-2 different sized sifters, triple beam balance, 100mL graduated cylinder, 4-5 baby food jars, timer, wet erase marker, 1 large jar or plastic bottle with lid. Procedures: I. Separating the sediments by size 1. Measure 100 mL of the mixed sediments into a beaker. 2. Make 3 massing boats using the aluminum foil by folding up the sides. Find the mass of each boat now and record on the data table. You will use this to hold the different samples of sand while finding the mass. 3. Sift the sediments into their assigned aluminum boat using two different sifters. Use the sifter with the smallest holes first, then whatever doesn’t go through the holes should be placed in the larger sifter. The particles that cannot go through either sifter are sample #3. 4. Draw a picture of one piece of the sediment in the data table. Draw it the same size as your sample. 5. Mass 30 mLs of each type of sand and subtract the mass of the ‘boat’. Record the data. Sample 1 Smallest sediment 2 Sediment 3 largest sediment DATA Table: Mass of Samples Drawing of Mass of boat (g) Mass of boat and Approximate sample (g) Particle Size Mass of Sample (g) II. Find the density of the samples 6. Pour sediment 1 into a graduated cylinder. Add 50 mLs of water and write down the new volume. Subtract the first volume from the second and you will know the volume of the air in the sediment. Subtract the amount of air from the original volume of the sediment. 7. Find the density of the sample by dividing the mass by the volume. DATA CHART Density of Samples Sample A Volume of dry sand (mL) B Volume of water to be added C Total sand and water A+B D Water and Sand shaken together E Air C-D F Volume (Sand without air) G Density M/V A-E 1 2 3 III. Can you identify the layers of sediment? 8. Add 50 mL of the original sediment from the supply bucket to a baby food jar. Add 50 mLs of water. Tighten the lid and shake. Set the jar down and observe the mixture. After five minutes draw the different layers. Label any layers that might coincide with the three layers you sorted. Draw and label the jar after settling: Analysis Questions: (Write the question and the answer, unless you know how to write a complete sentence.) 1. How many sizes of sediments were in your sample of sand? Do you think there could be more? Explain. 2. Which sample was most dense? Which was least? 3. Which sample was largest? Was it also the densest? Explain why you think this is. 4. Which of the samples took the longest to settle in the jar? Why do you think this is? 5. Describe how the samples settled when mixed in the jar. Why do you think the samples sorted this way? 6. If the samples were all the same size, how do you think they would sort? Explain. 7. If the samples were all the same density how do you think they would sort? Explain.