hot and cold cans engineering project 1
advertisement

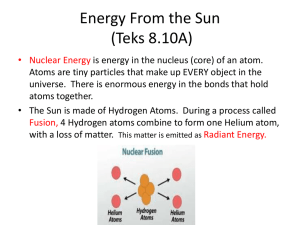
Learning Target I can design a solution to keep one can of 355 mL of 40C water warm as possible for 30 minutes and cool one 355 mL can of 40C water as much possible in 30 minutes using convection, conduction and radiation. Summary Students will apply the concepts of conduction, convection and radiation as they work in teams to solve two challenges. One problem requires that they maintain the warm temperature of one soda can filled with water at approximately human body temperature, and the other problem is to cause an identical soda can of warm water to cool as much as possible over a 30-minute time period. Students design their engineering solutions using only common everyday materials, and test their devices by recording the water temperatures in their two soda cans every one minute. Engineering Connection Engineers encounter problems of warming and cooling liquids in many situations. For prepared beverages, this might require maintaining specific cold or hot temperatures, but either way, the principles applied are the same. Students approach the activity challenges as if they were engineers, using heat transfer principles to achieve their goals. Pre-Requisite Knowledge Students will use their notes taken in class to facilitate their understanding of the concepts of conduction, convection and radiation. Students will use their notes to identify the type of energy transfer taking place and describe how it promotes heating and cooling for their cans. Learning Objectives After this activity, students should be able to: Describe everyday examples of ways people try to cause or prevent heating and cooling by conduction, convection and radiation. Give examples of materials that serve well for mechanisms of conduction, convection and radiation. Materials List Each pair of students need: 2 clean, empty, 12-ounce soda cans, ideally all the same type, plus 2 extra cans for controls laboratory thermometer (liquid immersion; accurate to 0.5 or 1°C) teacher supplied an assortment of "useful junk," such as fabric scraps (various sizes), socks from the lost & found, packing peanuts of several types, pieces of foam (various sizes), construction paper (both light and dark colors), bubble wrap, newspapers, quilt batting, old overhead transparencies, rubber tubing, drinking straws, funnels, aluminum foil, large zipper-type plastic bags, and anything else that might be used as insulating or conducting material, or to absorb or reflect radiation scissors, if possible glue, one bottle, if possible roll of tape, if possible (optional) timer or stopwatch Introduction Today you and your team will be acting as if you were engineers by applying your understanding of the concepts of conduction, convection and radiation to a design challenge: To design and build devices that keep one can of water as warm as possible, while cooling another can of water as much as possible. The cans will be filled with water that is at about 40 °C, which is very close to human body temperature. For 30 minutes, you will monitor the temperatures in each of your cans, recording the temperatures every one minute. At the end of the 30 minutes, you will determine how much the temperature changed in each of the cans. If you really understand what you learned about conduction, convection and radiation, then the temperature in one of your cans should change very little, and the temperature in the other can should decrease a lot. Vocabulary/Definitions conduction:The transfer of heat by molecular motion through a solid or a liquid, from a region of high temperature to a region of lower temperature. convection: The movement of heated molecules of a gas or a liquid from a heat source to another area, due to density differences within the gas or liquid. radiation: The transfer of heat energy by waves of visible or infrared light moving through space. Limitations/Constraints for Design Requirements No flame No water in solid or gas form (ice or steam) If water is used, it must be room temperature (app. 22C) No more than 1 linear meter of any type of material No human-made containers or devices (such as hand warmers, insulated lunch boxes, thermoses, hot pots flashlights, classroom radiators or air conditioners) No pouring out and replacing it with hot or cold water from the tap! The original water must stay in the can for the duration of the experiment Procedure Part 1: Designing the warming and cooling devices 1. Assemble all the "useful junk" materials, as well as tools (scissors, tape, glue) at a table. 2. Let students examine the materials, and then, working in teams of two students each, give pairs 20 minutes to brainstorm, plan and construct their cooling and warming devices. 3. Allow students to use empty cans during the planning and construction phases, which they will need to be able to remove and replace with filled cans for the testing phase. Part 2: Keeping cans warm and letting them cool 1. 2. 3. 4. Develop a data table to record temperatures When all groups are ready to test their warming-cooling devices, issue the water-filled cans and thermometers. Record the temperature of the water in the can every minute for 30 minutes. Record the temperature in 2 control cans every minute for 30 minutes. These will have no modifications made to them. Part 3: Data analysis and results presentations 1. Calculate the total change in temperature. 2. Graph the Time (x-axis) vs. Water Temperature (y-axis) on a line graph. 3. Use three colors for the lines, one color for warming device, one color for cooling device, and the third color for the control can. 4. Graph all three lines on one graph. 5. Each group will make a brief PowerPoint presentation to the class, in which team members present their graphs and summarize the performances of their warming and cooling devices by calculating the temperature change of each during the 30 minutes and comparing these to the temperature changes in the control cans. 6. As part of their presentations, team members should also show their warming and cooling devices or arrangements 7. Explain how pairs either tried to take advantage of conduction, convection and radiation, or how they tried to eliminate them. Investigative Questions These questions must be addressed in the PowerPoint presentation: Is this arrangement intended to help your can stay warm (or get cool) by conduction (or convection or radiation)? How does it do that? What was the total water temperature changes? How does the temperature in your can compare to the temperature in the control cans? Does that mean that your arrangement is working (to keep your can warm, or help your can get cooler)?