Building Your Own Balance
advertisement

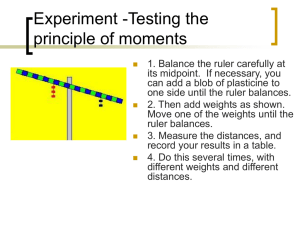
Name: _________________________________________ Date: ____________________ Building Your Own Balance In order to earn a 4 in the daily work category for science you need to complete a minimum of 5 extra credit activities per semester. This is due by the end of the semester however; it may be turned in at any time. In science class we use a triple-beam balance or an electronic balance to measure the mass of objects. In this activity you will build a single-beam balance to determine the masses of a penny, nickel, dime, and new state quarter. You will use the density of water to determine the mass needed to balance each object. These balances are surprisingly accurate when used properly. This activity will instruct you on how to make one type of balance; however, there are numerous designs for homemade single-beam balances. Feel free to substitute another design if you prefer. Materials: 1) 2) 3) 4) 5) 6) 7) 8) 2 small cups (preferably plastic) Ruler or pant-stir stick Duct tape or another way to attach the cups to the ruler Pencil or pen Syringe or medicine eye dropper (some way to measure water to 0.1 of a gram) Clay (optional) Penny, nickel, dime, and new state quarter Something that will take a picture (camera, phone, etc) Procedure: 1) Attach a cup to each end of the ruler. 2) Balance the ruler (cup side up) on the pencil or pen placed under the middle of the ruler. Using a hexagonal pencil will hurt the accuracy a bit, but using a round pencil makes it difficult to get the balance to stabilize. Choose which one will work best for you. 3) Make adjustments in the balance point by adding small extra pieces of tape (or clay) until the ruler is balanced. a. When balanced the ruler may not be perfectly level. You will be able to tell it is balanced because tapping it will cause it to sway to either side. When the ruler is not balanced, it will only sway to one side. b. You should check the empty balance each time before you measure something new and calibrate it with small pieces of tape if needed. 4) Put the object you want to find the mass of in one cup. 5) Use the syringe to fill the other cup with water until the single-beam is in balance again. 6) Measure and record the volume of water you used. Remember that the density of water is 1.0g/mL and that 1 mL=1cc. That means that 1 mL or cc of water has the mass of 1 gram. If it takes 27mL to balance out the object, then the mass of the object is 27grams. 7) Take a picture of your homemade single-beam balance in a balanced position. Attach your picture to this lab when you turn it in for credit. Data: Please find the mass of the objects listed in the data table below. Object Volume of Water (mL or cc) Mass (g) Penny Nickel Dime New State Quarter An object of your choosing: Data Analysis: 1) What was the percentage difference between your measurements and the accepted masses (provided by your teacher)? a. Percent Difference = ([difference between the two answers] ÷ [accepted answer]) x 100 2) What do you think was the biggest contributor to that difference? Conclusion: Please answer these questions in complete sentences. 1) Did you use a hexagonal or round pencil/pen? Why? 2) How are weight and mass different? 3) Explain why a balance measures mass while a scale measures weight? 4) What do you think is the least amount of mass you can accurately measure with your balance? Why? 5) What would happen if you used salt water or root beer instead of water in your balance?



![Measuring Reaction Times (modified) [word document]](http://s3.studylib.net/store/data/005890593_1-f3403f0a4fee937a93ebd23d2df416ab-300x300.png)