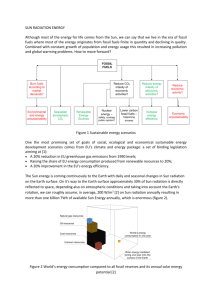
Efficiency, output, input , rate and specific energy
advertisement
TOPIC 8 ENERGY PRODUCTION NEW SL and HL CORE 1 Read Tsokos pp. 415 – 429 ( also scanned on Moodle) and do the following questions. May need to use internet sources outside the book to answer some of the questions. Units : Power, J ,MJ, W, MW kWh, eV = 1.6 x 10-19 J Power = Work = energy per second t P = Fd t P=Fv v =d t Watt ( W) = Js-1 1 MJ = 1 x 106 J ( 1 million J) 1 eV = 1.6 x 10-19 J 1MeV = 1 million eV 1 MW = 1 x 106 W ( 1 million W) 1GW = 1000 MW The Kilowatt hour: Defined as the energy used in kilowatts in one hour. 1kW = 1000 W = 1000 Js-1 1kWh = 1000 Js-1 x 3600 s ( in 1 hr) = 3.6 x 106 J 1kWh = 3.6 x 106 J IN Data Booklet 2 Example 1 A power plant produces 500 MW of power. a) How much energy is produced in one second? Express your answer in (i) j (ii) kWh b) How much energy in joules is produced in one year? Check answers: a) 5 x 108 J , 140 kWh b) 1.6 x 1016 J 1. Energy degradation and Power generation Tsokos pp. 415-433 Energy degradation, production and sources pp. 415 – 418 Read and do the following. May need to use internet sources outside the book to answer some of the questions. 1. Definitions : a) Sankey diagram – diagram that represents energy flows b) Energy degradation – excess energy lost and is “ less useful” and can not be used to perform mechanical work c) Non- renewable energy source ( give 2 examples)– finite sources that will run out e.g : fossil fuels, nuclear d) Renewable energy source ( give 3 examples ) - energy that can renew itself eg. : solar, wind, wave - tidal, geothermal, hydroelectricity e) Energy density ( state unit) – energy , in joules (J), that can be obtained from one cubic meter m3 : Jm-3 . High energy density = high power output f) Specific Energy – energy , in joules (J), that can be obtained from one Kg : Jkg-1 . 3 2. Answer these questions Example 2: Degraded energy is energy that is A. stored in the Earth’s atmosphere. B. available from non-renewable energy sources. C. converted into work in a cyclical process. D. no longer available for the performance of useful work Example 3: Power plants Efficiency = output energy input energy Power plant problems basically deal with how much useful energy that they can produce ( output) to be used as electricity based on how much energy they are using ( input) to produce that energy. Power plants input energy sources are usually coal ( fossil fuels) , nuclear or hydro. ( water dams) and the challenge is to get away from these and use renewable sources : solar, wind, geothermal and more hydro. 4 Example 4: Explain fig. 1.1 below and why you only get 25% efficiency rating using the formula for efficiency . Also, explain how much is considered degraded energy: Efficiency = output energy input energy 5 Efficiency, output, input , rate and specific energy: Need input energy in order to produce output energy and you never get 100 %. For example , nuclear reactors take huge amounts of energy from nuclear reactions and take what’s left over ( output) to send to cities and homes Efficiency = output energy Input energy Rate = input energy Specific energy Rate = rate of input energy that is being used up to produce output energy. For example the rate that a power station is using up its fuel, natural gas, to produce electricity – its output energy. Specific Energy – the amount of energy that can be obtained from 1 kg of substance. Example 5: a) A power station has an output power of 500 MW and overall efficiency of 27%. It uses natural gas as a fuel. How much input energy is being consumed by the natural gas? b) Natural gas has an specific energy of 56 MJ kg-1. 1 MW = 1 MJs-1 . Calculate the rate of consumption of natural gas in the power station in kgs-1 by using the specific energy and the input energy ( in MW) calculated in part a above. Rate = input energy Specific energy 6 Rate vs. input Both indicate how much energy is used over a certain period of time. The only difference is the units : rate is in kgs-1 and input is in W = Js-1. Example 6: A power plant produces electricity by burning coal, using the thermal energy produced as input to a steam engine, which makes a turbine turn, producing electricity. The plant has a power output of 400 MW and operates at an overall efficiency of 35%. a) Calculate the rate at which thermal energy is provided by the burning coal. i.e calculate the energy input that the burning coal provides for the power plant. b) Calculate rate at which coal is being burned in kgs-1. Specific Energy of coal is 30MJkg-1. 7 Example 7: A coal burning power plant produces 1.0 GW of electricity. The overall efficiency of the power plant is 40%. Taking the specific energy of coal to be 30 MJkg-1 , calculate the amount of coal that must be burned in one day. Ans. : 7.2 x 106 kg day-1 8 KNOW BASICS of STEAM and COMBUSTION ENGINES 9 2. Fossil Fuels, Nuclear Power, Solar Power, Wind Power, Wave Power pp. 418 – 433. 1. Fossil Fuels Example 8: This question is about power generation. (a) Describe the origin of fossil fuels. (b) An electrical power generating station using fossil fuels as its source of energy has an output of 2 GW. It has been suggested that this station should be replaced by wind turbines, each providing 0.8 MW of electrical power. (i) State two advantages of the use of wind power ( Can not simply say pollution free). (ii) State and explain two disadvantages of using wind turbines to replace the fossil-fuel generating station. (c ) List 4 advantages of fossil fuels (d) List 3 disadvantages of fossil fuels 10 2. Nuclear Power pp. 420 – 423 Fuel is typically U – 235 n1 + 235 U 236 U 140 Xe + 94 Sr + 2 1 n 0 92 92 54 38 0 Neutron must bombard U 235 to initiate rxn. The rxn is self sustaining and is called a chain rxn. For rxn to keep going a certain minimum number of U 235 must be present otherwise the neutrons escape without causing further rxns. – this is called critical mass. U 235 will only catch the neutrons if they are not moving too fast. The neutrons that are produced during the rxn are too fast so they have to be slowed down. The Moderator, Control Rods and Coolant: Slowing down the neutrons is achieved by collisions of neutrons with a moderator which is a material surrounding the fuel rods. The moderator is usually water or graphite. The fuel rods are the tubes that contain 235. NOTE: if water is the moderator, do not confuse with the other water that is used as a coolant. The moderator does not cool down the reaction AND the coolant does not slow down the reaction If the rxn gets out of hand, i.e too many neutrons flying around, control rods are used. These absorb excess neutrons when the rxn begins to get out of hand and are introduced by the engineer- tech. when needed. An atomic bomb has no control rods. 11 Example 9 : This question is about nuclear power. (a) A fuel often used in nuclear reactors is uranium. Explain why uranium is a non-renewable energy source. (b) One nuclear reaction that takes place in a reactor is 1 n + 235 U 236 U 140 Xe + 94 Sr + x 1 n 0 92 92 54 38 0 (i) State the number x of neutrons produced in this reaction. (ii) Using the equation explain what is meant by a chain reaction. (iii) Explain how, in a reactor, the production of energy in a chain reaction is controlled. (c) Outline how the energy produced in fission reactions is transferred to thermal energy. (d) State one advantage of nuclear power production compared to fossil fuel power production. (e) When a uranium nucleus fissions, approximately 180 MeV of energy is released. The overall efficiency of a nuclear reactor is 23 and its output power is 450 MW. Calculate the number of fissions required per second: 12 Example 10: Example 11: Example 12: Critical mass refers to the amount of fissile material that A. will allow fission to be sustained. B. is equivalent to 235 g of uranium. C. will produce a growing chain reaction. D. is the minimum mass necessary for fission to take place 13 Example 13: The commercial production of energy by nuclear fusion is not yet possible mainly due to difficulties with A. obtaining plentiful supplies of a suitable fuel. B. reaching the high temperatures required. C. confining the hot plasma. D. disposing of the radioactive waste products. Example 14 : List 3 advantages and 4 disadvantages of nuclear energy : 14 3. Solar Power pp. 423 – 425 1. Define and explain : a) Active solar devices : 15 b) Photovoltaic cell : List 3 advantages and 4 disadvantages of solar energy : 16 Example 15: This question is about solar power. (a) Describe, in terms of energy transformations, the difference between a photovoltaic cell and an active solar heater. (b) A photovoltaic cell of area 6.5 10–4 m2 is situated on the roof of a house. The cell has an efficiency of 8%. At a time when the power of the solar radiation incident on the photovoltaic cell is a maximum, the energy of a cell delivers an input power of 47 W . (c) (i) Calculate the amount of power output per square meter of 1 photovoltaic cell delivered after loss due to the efficiency rating. (ii) State one reason why the power of solar radiation at any particular region does not have a constant value. A power of 30 kW is required to produce adequate hot water for the house. (i) Use the data from (b) to determine the minimum number of photovoltaic cells required to generate this power. (ii) State and explain whether it is more practical to use photovoltaic cells or an active solar heater to provide hot water for the house. 17 4. Hydroelectric, Wind and Wave Power pp. 425-429 Hydroelectric fig. 1.14 Power generated by hydroelectricity depends on how much water can drop from a certain height over time. It requires large volume flow rates ( Q) and large heights ( h) and basically depends on how fast and from how high water flows and drops. Gravity and density are constant. Power formula for Hydroelectricity P = pQgh units in W p = density of water = 1000kgm-3 Q = volume rate in Ls-1 ( 1 L = 1kg) g = gravity = 9.8 ms-2 h = height in m Example 16: Find the power developed when water in a stream with flow rate 50Ls-1 falls from a height of 15m. Check Ans. 7.4 MW = 7.4 x 106W 18 Example 17 : Example 18 : List 3advantages and 3 disadvantages of hydroelectricity. 19 Wind Example 19 : This question is about wind power. The maximum theoretical wind power P for air of speed v moving normally through area A where is the density of air is given by P (a) ρ Av 3 . 2 Units = W (i) Air of density 1.3 kg m–3 and speed of 9.0 m s–1 is incident on a wind turbine having blades of diameter 15 m. Calculate the maximum wind power incident on the turbine. (ii) State why it is impossible in practice to extract all of the power P in (i) from the air. (iii) State two reasons why wind turbines are not placed close to one another. Wave List 3 advantages and 6 disadvantages of wave power. 20 3.The Greenhouse Effect and Global Warming pp. 433– 453 . Focus on these notes only. 1. Black body law / Stefan – Boltzmann Law: emitted radiation Any body in the universe will radiate energy in the form of electromagnetic radiation. The Stefan – Boltzmann law states that the amount of energy per second or power (P) radiated by a body depends on the surface area (A), absolute temperature (T), and the properties of the surface called emissivity ( e) : P = e σ AT4 T emp. Must be in K . If given in C convert to K : K = C + 273 Where σ is the Stefan-Boltzmann constant = 5.67 x 10-8 Wm-2 K-4 Emissivity ( e) and Black body – do not confuse emit with reflect Emissivity describes the ability of a body’s SURFACE to emit radiation. Dark and dull surfaces emit better than light and shiny surfaces. The black body is a theoretical body that is a “ perfect” emitter of radiation. The values of e range from 1 to 0 with 1 being the perfect emitter. There are no units ( similar to an index for example the index of refraction) Some examples: Black body e=1 Ice e = 0.1 ( good emitter and absorber) ( not a good emitter but a good reflector) Surfaces that are black and dull , as opposed to light and shiny, are also good absorbers of radiation. Thus we wear dark clothes in the winter to absorb the radiation. White T shirts are good reflectors of radiation which is why people wear them during hot summer days (Arabians use white clothing in desserts). 21 Temperature and Wavelength As temperature increases, energy increases, frequency increases and wavelength decreases. Questions Example 20 : By what factor does the power emitted by a body increase when the temperature is increased from 1000 C to 2000 C ? Check Ans. 2.5- 2.6 acceptable range correct Example 21 : By what factor does the rate of radiation from a body increase when the temperature is increased from 500 C to 1000 C ? Check Ans 1.8 22 Intensity vs. Wavelength Graphs – Black Body Spectra With increasing temperature the peak of the curve occurs at higher intensities and slightly shorter wavelengths. This makes sense because higher temperatures = higher frequencies and shorter wavelengths. If you reverse this graph and put intensity on the x –axis and wavelength on the y- axis. Black bodies with higher temperatures should peak at a shorter wavelengths AT EVERY POINT ON THE GRAPH. SEE example 22 below: Example 22 Two black bodies X and Y are at different temperatures. The temperature of body Y is higher than that of body X. Which of the following shows the black body spectra for the two bodies? 23 2. The Albedo – α : radiation reflected or scattered The albedo of a body is defined as the ratio of power of radiation reflected or scattered from the body to the total power incident on the body: α = total scattered OR reflected power total incident power total reflected radiation = total incident – total absorbed Bodies with high reflective surfaces have a high albedo. Snow, for example , has an albedo of 0.85 ( no units = type of index) indicating that snow reflects most of the radiation that hits it ( incident) whereas charcoal has a low albedo of only 0.04 , meaning that it reflects very little light incident on it. Albedo are always lees than 1.0. Also remember that snow and ice are poor emitters of radiation emissivity = 0.1 Example 23 : Calculate the average albedo of earth if the average incident solar radiation is 350 Wm-2 and the average absorbed radiation is 250 Wm -2. Global Warming A HIGH albedo, mainly due to the polar ice caps, is important to avoid global warming. This means that the earth will reflect much of the incoming radiation back out , If the polar ice caps melt the earth’s albedo decreases. 24 Example 24 : The diagram below shows a simplified model of the energy balance for Earth. The albedo of the Earth according to this model is equal to A. 2 . 340 B. 100 . 340 C. 238 . 340 D. 240 . 340 25 3. Greenhouse effect – Basics The greenhouse effect is the warming of the earth. It is caused when short wave UV radiation from the sun is transmitted in through the atmosphere and other green house gases. However , the radiation is reemitted as infrared radiation by the earth’s surface, which is absorbed by various gases in the earth’s atmosphere. The infrared radiation is then partly radiated back towards the surface of the earth. In other words the UV radiation from the sun can get in to the earth’s atmosphere but the infrared radiation can not get out. The gases primarily responsible for this effect are : water vapor, carbon dioxide , methane and nitrous oxide. Global warming Due to the greenhouse effect and human activities which have been increasing the concentration of greenhouse gases the earth is undergoing global warming. 26 The change in sea level is always varying but the present value is about 100m more than the last ice age 18 000 years ago. Changes in sea level affect the amount of water that can evaporate and the amount of thermal energy that can be exchanged with the atmosphere. In addition, changes in sea level affect ocean currents. The presence of these currents is vital in transferring thermal energy from the warm tropics to colder regions. Example 25 : It is hypothesized that global warming may lead to significant changes in the average sealevel. This hypothesis assumes that A. average rainfall will increase. B. icebergs will melt. C. glaciers will melt. D. the rate of evaporation of seawater will increase. OK 27