Experement 3 Marcet boiler
advertisement

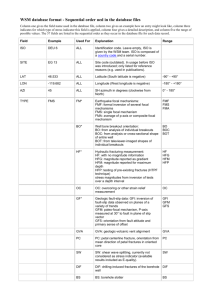
The University of Jordan Faculty of Engineering and Technology Thermodynamics Lab Report Expirement No.2 Instructor: Eng. Rebhi Al-Mashaleh Students Names: Fareed Shatara 2110302 فريد ماهر شطارة Hanna Mansour 0128358 حنا سليم منصور Ghassan Hjazi 0127296 غسان حجازي Ahmad Abu Malloh 0127293 أحمد ابو ملوح Omar Al Khateeb عمر الخطيب 0120533 Section: 2 Date: 16 / 03 /2014 Objectives: To Investigate The Relationship Between The pressure And The Temperature For a Saturated Steam in a Constant Volume Tank. And To verify the Clausius – Claperon Equation. Apparatus : Gunt Marcet Boiler Theory: 𝐝𝐓 𝐝𝐏 = 𝑻.𝑽𝒇𝒈 𝒉𝒇𝒈 T = Abs. Temp. Vfg = Vg – Vf Hfg = hf – hg where : Results and Calculations: Gauge Pressure (bar) 0 1 2 3 4 5 6 7 8 9 10 Absoulte Pressure 0.9 1.9 2.9 3.9 4.9 5.9 6.9 7.9 8.9 9.9 10.9 Graph & Plotting : Steam Temp . Increasing Pressure 95 115.2 129.5 140 148.6 156.3 161.9 168.1 172.5 177.1 182.2 Table(1) Steam Decreasing Pressure 97.5 117.7 132 142.5 150.3 157.5 163.9 169.5 174.7 179.6 182.2 Mean Temp. 96.25 116.45 130.75 141.25 149.45 156.9 162.9 168.8 173.6 178.35 182.2 Graph Eqaution : Using Matlab we Get This Equation y = p1*x^2 + p2*x + p3 Coefficients: p1 = -6.9843e-05 p2 = 0.16191 p3 = 86.397 Sample Of Calculations : - Theoretical dT/dP : The clausius-claperon equation was used for calculating the theoretical dT/dp : ( dT ) (dP )sat = T . Vfg hfg The calculations of the point of pressure equals 3.9 bar is briefly shown here : Table (2 ) : Saturated Water and Steam Table P (bar) 3 3.9 4 T (C ) 133.5 T* 143.6 By interpolation we get : T* = 142.59 oC Vg* = 0.4766 m3/kg hfg = 2137 kj / kg Then apply clausius-claperon equation : ( dT ) (dP )sat = (142.59+273 ) . (0.4766) 2137 ( dT ) / (dP )sat = 0.09269 kelvin / kpa Vg 0.6057 Vg* 0.4623 hfg 2164 hfg* 2134 -Experimental dT/dP : The experimental dT/dP represents the slope of T-P diagram , where ( T in kelvin , and P in kpa ), the slope was estimated by fitting the curve to 2nd degree polynomial , then differentiate the fitting function , these procedure was done by matlab : T(p) = p1 . (p2) + p2 . (p) + p3 dT dP = 2(p1) . p + p2 dT =( 2) . ( - 6.9843 . 10-5 ). (390) + (0.16191) dP dT = 0.10743246 Dp -The error's : The error can be simply estimated by the following formula : Error % = theoretical value - experimental value x 100% Theoretical value The calculations of the point of pressure equals 3.9 bar is briefly shown here : Error % = 0.09269 - 0.10743246 0.09269 x 100% Error = 15.9 % Discussion and Conclusion : Marcet boiler is the device which we use to study the relation in between pressure and temperature for a water at saturated liquid phase. As we did in the laboratory, we started heating water with constant pressure until it reached boiling point. Then, closing the valve which created a constant volume system. Forcing the pressure to increase as the temperature rises. And thus studying the direct relation between pressure and temperature for water at that point. We notice that it is essential to close the valve as we reach boiling point to make sure we are now in a constant volume process, otherwise pressure would have never increased. Causing the experiment to be useless. We also notice that we closed the valve exactly when we reached boiling temperature (95 c at 0.9 bar pressure) and thus keeping water at saturated liquid phase. After studying the results and plotting the diagram we find that the relation in between pressure and temperature is directly proportional. The difference between the theoretical values and the actual values is caused by errors with certain calculated acceptable percentages.