Male & Female HRT Risks, Successes, the Future? Teresa Bailey
advertisement

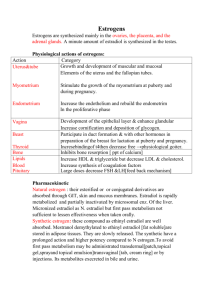
1 Male & Female HRT Risks, Successes, the Future? Teresa Bailey, PharmD, BCPS, BCACP, FCCP June 2015 Objectives Explain the association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. Discuss the potential benefits of hormone replacement therapy in women Discuss the pros and cons of dehydroepiandrosterone for peri- and postmenopausal women. Pathophysiology Menopause o Loss of ovarian function leading to a state of permanent amenorrhea o Ovaries no longer able to respond to pituitary stimulating hormones, so estrogen levels are low and production of FSH and LH increases dramatically. o Average age of onset-51.4 years o Onset unaffected by: race, socioeconomic status, alcohol consumption, age of menarche, or age of last pregnancy. Cigarette smoking may accelerate onset up to 2 years because of gametotoxic effects of cigarette smoke on steroid hormone metabolism by liver. o Premature menopause-loss of ovarian function before the age of 35 years due to ovarian surgery, endocrinologic, autoimmune disorders 2 Estrogen and Progestin Therapy Benefits and Risks of Estrogen and Progestin Therapy (Ann Intern Med 1992;117(12):10381041) Benefits Estrogen 1. Relieves genitourinary atrophy Thinning of hair of the mons and shrinkage of the labia minora Atrophy of vulva leads to pruritus and pain Vaginal pH 4.55 68 creating a favorable environment for bacterial colonization Loss of lubrication dyspareunia Recurrent episodes of urinary frequency and urgency with dysuria 2. Relieves vasomotor instability Occurs in 75%–85% of women. Occurs usually within 12–24 months after the last menstrual period. Increased skin temperature, nausea, dizziness, headache, palpitations, diaphoresis, night sweats. 3. Osteoporosis-Reduction of hip fractures by 25%. Reduction of vertebral fractures by 50%. Estrogen reduces rate of resorption but does not restore bone loss. Progestin 1. Decrease risk of estrogen-induced irregular bleeding, endometrial hyperplasia, and carcinoma 2. Protection against breast carcinoma 3. Enhancement of estrogen prophylaxis of osteoporosis 4. Lowers LDL cholesterol by 11% and increases HDL cholesterol by 10%. However, was not shown to lower coronary heart disease based on HERS trial. 5. Increase in life expectancy 6. Insomnia and fatigue 7. Mood changes 8. Sexual function Risks Estrogen 1. Endometrial cancer by unopposed estrogen. Duration of estrogen use dependent; eightfold for 10–20 years of use. Recommended not to use in women with a history of endometrial cancer. 2. Breast cancer with unopposed estrogenuncertain. May increase slightly (25%) among women who take estrogen for 1020 years. Recommended not to use in women with a history of breast cancer. 3. Increased risk of cardiovascular outcomes. (See WHI trial). 4. Adverse effects: bloating, headache, breast tenderness (5–10%) 5. Unpredictable uterine bleeding with unopposed estrogen (35–40%) Progestin 1. Adverse effects: bloating, weight gain, irritability, depression. (Dose related) 2. Unpredictable endometrial bleeding with continuous estrogen/progestin during first 8–12 months (30–50%) 3 Contraindications of HRT • • • • • • • • • Undiagnosed abnormal genital bleeding Known, suspected, or history of breast cancer Known or suspected estrogen-dependent neoplasia Active DVT, PE, or a history of these conditions Active arterial thromboembolic disease (stroke and MI), or a history of these conditions Known anaphylactic reaction or angioedema to estrogens/progestins Known liver dysfunction or disease Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders Known or suspected pregnancy Efficacy on Coronary Artery Disease (JAMA 1998;280:605-613) WHI Trial: Efficacy on Coronary Artery Disease (NIH News Release NHLBI Stops Trial of Estrogen Plus Progestin Due to Increased Breast Cancer Risk, Lack of Overall Benefit. www.nhlbi.nih.gov/new/press/02-07-09.htm) Objective: HRT alter risk for coronary heart disease in postmenopausal women with established coronary disease Methods o R, DB, PC, 20 US center. Follow-up x 4.1 years o N=2763 women < 80 years old with established coronary disease o Treatment: Prempro 0.625/2.5 QD versus placebo Results o Baseline characteristics similar o Cardiovascular Outcomes Outcomes Primary CHD events 29% increase Stroke/TIA 41% increase Deep vein thrombosis 50% increase Breast cancer 26% increase Hip fracture 33% decrease Any fracture 24% decrease Colorectal cancer 37% decrease Longer duration of use greater in relative hazards in nonfatal MI and CHD death Hormone Therapy for Preventing Cardiovascular Disease in Post-menopausal Women. Cochrane Review 2015 19 studies reviewed N = 40,410 post-menopausal women For primary or secondary prevention of cardiovascular disease events Results 4 o o No protective effects for all-cause mortality, cardiovascular death, non-fatal myocardial infarction, angina, or revascularization Increased risk Stroke (NNH = 165) Venous thromboembolism (NNH = 118) Pulmonary emboli (NNH = 242) Greater risk when taking > 10 years Benefits and Risks of Estrogen and Progestin Therapy Ann Intern Med. 2013;158:47-54. 5 Treatment Algorithm of Menopause Pharmacotherapy: A Pathophysiologic Approach, 9e Joseph T. DiPiro, Robert L. Talbert, Gary C. Yee, Gary R. Matzke, Barbara G. Wells, L. Michael Posey 6 Conjugated Estrogens/Bazedoxifene (Duavee) • Indications o Treatment of moderate/severe vasomotor symptoms associated with menopause o Prevention of osteoporosis in postmenopausal women with a uterus • Bazedoxifene: SERM o Reduces risk of endometrial hyperplasia • Dose: 0.45 mg/20 mg daily • Similar precautions to estrogen/progestin • Amenorrhea at 1 year: 83-88% Bioidentical Hormones “Natural” identical to hormones in the body Claim to be safer than prescription hormones Patient and provider testimonies Estradiol -More stimulating to breast Estrone -Stimulating to breast Estriol -More active on vagina, cervix, and vulva; Least stimulating to breast Side Effects o May have less side effects than synthetic estrogens o Breast tenderness/fullness, edema, headaches Dosage o Estrace 1 mg daily o Estraderm patch 0.05 mg twice weekly o Estrone 10%, estradiol 10%, estriol 80% o Estriol cream 0.5mg/gram Testosterone for peri and postmenopausal women. Cochrane Database of Systematic Reviews 2005 • Whether testosterone is safe and effective to add to HRT for postmenopausal women • 35 trials (4768 participants) • Variety of testosterone formulations • Duration was six months (range 1.5 to 24 months) • Results o Sexual function scores improved o Number of satisfying sexual episodes increased o Adverse effects HDL cholesterol levels decreased Hair growth and acne increased DHEA • • Purported Use o Anti-aging, anti-cancer, anti-obesity, cholesterol, arthritis, fatigue, diabetes, Alzheimer’s, AIDS, osteoporosis Mechanism of Action o Adrenal steroid that is precursor to other steroids (estrogen, androgen) o Has no estrogenic and androgenic effects 7 • NEJM 1999;341:1013-20. o Randomized, double-blind, placebo, crossover o 24 women with adrenal insufficiency o DHEA 50 mg QAM x 4 months o Steroid and sex hormones DHEA, androstenedione, testosterone, dihydrotestosterone increased to normal levels* Estrone and estradiol: no change o Serum Lipids Total cholesterol and HDL: decreased* LDL and triglycerides: no change o Well-being 90-Item Symptom Checklist for depression, anxiety, obsessive-compulsive, hostility* Multidimensional Mood Questionnaire improved* Hospital Anxiety and Depression Scale improved* o Sexuality Frequency of sexual thoughts* Degree of sexual interest* Level of mental satisfaction with sex* Level of physical satisfaction with sex* DHEA for Women in the Peri- or Postmenopausal Phase. Cochrane Review 2015 Evaluate the safety and efficacy of DHEA to women with menopausal symptoms in the peri- or postmenopausal phase o 28 trials o N = 1,273 menopausal women o Compared to placebo No improvement of quality of life Increased androgenic side effects (acne) Unclear whether DHEA affected menopausal symptoms Improved sexual function Similar results with oral or topical o Insufficient data to compare to HRT Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebocontrolled trials.J Clin Endocrinol Metab. 2013 Sep;98(9):3615-26 o 25 studies (1353 elderly men) o Mean duration 36 weeks o Results No effect of DHEA supplementation in comparison with placebo Lipid and glycemic metabolism Bone health Sexual function Quality of life Contraindications/Precautions o Breast, uterine, or ovarian cancer o Prostate cancer o Pregnancy/Lactation o Liver impairment 8 o Diabetes (increased insulin resistance) o Hyperlipidemia (decreased HDL) o Polycystic ovary syndrome may worsen Drug Interactions o Aromatase inhibitors Anastrozole, exemestane, letrozole DHEA may interfere o Corticosteroids (suppress DHEA production) o Fulvestrant [Faslodex] (DHEA may interfere) o Tamoxifen (DHEA may interfere) o CYP 3A4 Triazolam concentrations increase o Insulin may decrease DHEA effectiveness Side Effects o Dose related o Masculinization in females o Voice deepening o Insomnia o Hirsutism or hair loss o Oily skin/acne o Menstrual irregularities o Headaches o Insulin resistance o Hepatic dysfunction o Abdominal pain o Hypertension o Mania o Long-term side effects are unknown o Study duration 1-2 yrs Testosterone Deficiency • • • • Other terminology o Androgen Deficiency Syndrome o Hypogonadism o Male Menopause Total testosterone level < 300 ng/dL Typically 1% decline/year after 30 years old 20% men > 70 years old: hypogonadism • Signs/symptoms 9 • Benefits o Improved libido o Improved mood and cognition o Reduced fat mass o Increased lean body mass o Increased body muscle strength o Increased aerobic endurance o Improved insulin resistance in DM o Increased BMD • Potential Risks o Acne o Male pattern baldness o Gynecomastia o Worsen sleep apnea (excessive doses) o Stimulate BPH symptoms o Affect metastatic prostate and breast cancers • Association of Testosterone Therapy with Mortality, Myocardial Infarction, and Stroke in Men with Low Testosterone Levels. JAMA 2013:310:1829-1836 o Retrospective, cohort study o 23,173 male veterans underwent coronary angiography and had testosterone level checked o Testosterone level 300 ng/dL o Rx for testosterone gel (1%), patch (63%), or injection (36%) o Association between TT with all-cause mortality, myocardial infarction, stroke o N = 8,709 men with Testosterone level < 300 ng/dL with or without CAD 10 o Primary endpoint Combined end point of time to all-cause mortality or to hospitalization for MI or ischemic stroke HR = 1.29 (CI, 1.05-1.58) Regardless of presence of CAD • AUA Position Statement o Potential for misuse of testosterone for non-medical indications, such as body building or performance enhancement o Testosterone therapy appropriate treatment for hypogonadism after full discussion of potential adverse effects o Testosterone therapy in the absence of hypogonadism not appropriate • Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010 Jun;95(6):2536-59 o Treatment of androgen deficiency with testosterone o Men with sexual dysfunction o Patients with chronic illness and low testosterone levels o Glucocorticoid-treated men o Older men with low serum testosterone concentration-not recommended • Conclusion o Risks may outweigh the benefits for Estrogen/progestin therapy Testosterone replacement therapy DHEA o Studies lacking Bioidentical hormones 11 Appendix. Products and Dosing of Estrogens and Progestins for Hormone Replacement Oral Estrogen Products Generic Name Conjugated estrogens, natural source Synthetic A Synthetic B Conjugated estrogens/medroxyprogesterone acetate Conjugated estrogens/medroxyprogesterone acetate Brand Name Premarin Cenestin Enjuvia® Prempro Premphase Dose 0.03–1.25 mg/da 0.625 mg/2.5 mg/day 0.625 mg/d for 14 days, then 0.625 mg/5 mg per day for 14 days Estrace generics Esterified estrogens 0.3–1.25 mg/da Menest Estropipate 0.75–6 mg/da Ortho-Est generics Estradiol/norgestimate One daily Prefest Estradiol/norethindrone acetate One daily Activella Mimvey generics Ethinyl estradiol/norethindrone acetate One daily Femhrt 1/5 Jintelli a May administer continuously or cyclically with 3 weeks of daily estrogen followed by 1 week off. Estradiol Vaginal Estrogen Products Generic Name Micronized estradiol Conjugated estrogens Estradiol Estradiol Brand Name Estrace Dose One dose daily x 2 weeks, then ½ dose daily x 2 weeks, then one full dose 1–3 times/week for 3 weeks 1.25–2.5 mg daily for 3 weeks One ring Q 3 months Premarin Estring Femring Vagifem Transdermal Estrogen Products Generic Name Estradiol patch Estradiol patch 0.014 mg Estradiol dot patch 0.0375mg, 0.05 mg, 0.075 mg, 0.1 mg Estradiol/norethindrone acetate Estradiol emulsion Estradiol gel Estradiol gel packet Estradiol gel Estradiol spray One vaginal tablet twice weekly Brand Name Alora Climara generics Menostar Vivelle Dot Minivelle Combipatch Estrasorb EstraGel Divigel Elestrin Evamist Dose 0.05–0.1 mg patch twice weekly 0.014 mg patch weekly One patch twice weekly One patch twice weekly Apply 3.48 g to skin daily Apply 1.25 g daily Apply 1 packet daily Apply 1-2 pumps daily Apply 1-3 sprays daily 12 Oral Progestin Products Generic Name Medroxyprogesterone acetate Norethindrone Acetate Progesterone Brand Name Provera Cycrin generics Aygestin generics Prometrium generics Dose 5–10 mg daily for 5 to 10 days 2.5–10 mg daily for 15 days 100mg 2 daily for 12 days 13 Dosing and Products of Testosterone Replacement. Colquitt CW, Robertson GL. Testosterone Deficiency. US Pharm 2012;37:34-8.