News Release
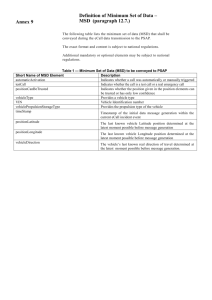
FOR IMMEDIATE RELEASE
Media Contact:
Matt Mellor
Tel: 01694 731777
matt@abccomms.co.uk
Nationwide diagnostics confirm continuing leptospirosis threat
MILTON KEYNES, UK, April 8, 2015 - Leptospirosis remains a common threat to dairy and
beef herds in Britain, according to extensive new data collected over the last 18 months from
the MSD Animal Health EXPERTIS™ BeefCheck and DairyCheck disease diagnostic
schemes.
This latest data set, taken from 1841 non-vaccinating farms1, shows that on average 45% of
herds were positive for leptospirosis antibodies, highlighting the importance of vaccination at
turnout. When looking specifically at dairy herds, the infection level rose to 58%.
The disease was prevalent in all parts of the country (see disease map), with over 50% of
the herds being affected in Wales and large parts of south west, northern and central
England.
“Leptospirosis is caused by a bacterial infection, and there are two strains which are known
to affect UK herds, L. hardjo prajitno and L. hardjo bovis,” explains MSD technical manager
John Atkinson. “In some cases, the effects of an infection can be dramatic. For example, in a
Use medicines responsibly. For more information visit: www.noah.co.uk/responsible
BOVILIS® BVD is an inactivated vaccine containing 50 ELISA units (EU) and inducing at least 4.6 log2 VN
units per dose of cytopathogenic BVD virus strain C86. Legal category: POM-V. BOVILIS BVD is only
available from your veterinary surgeon from whom advice should be sought. BOVILIS BVD is the
property of Intervet International B.V. or affiliated companies or licensors and are protected by
copyrights, trademark and other intellectual property laws. Copyright © 2014 Intervet International B.V. All
rights reserved.
For information regarding side effects, precautions, warnings and contra-indications please refer to the
datasheet at www.noahcompendium.co.uk. Further information is available from MSD Animal Health,
Walton Manor, Walton, Milton Keynes, MK7 7AJ. Tel: 01908 685 685 • vet-support-uk@merck.com •
www.msd-animal-health.co.uk
recent leptospirosis outbreak in Ayr reported in March this year2, 12 cows from a herd of 160
experienced a sudden onset drop in milk production.”
In many cases, however, the disease doesn’t cause these dramatic effects, but it can
significantly lower fertility and cause poor calf health3.
“Spring turnout is traditionally the time of year to make sure your herd is fully protected
against bovine leptospirosis,” he continues “This is because, at grass, uninfected cattle are
suddenly exposed to the urine of infected animals that may be shedding the disease. “Moist
spring grass is also a favourable environment for leptospirosis to survive outside the host.”
Leptospirosis also infects people, for example through contact with cattle urine. The latest
data from Public Health England4 shows that farmers are at particular risk of contracting this
infection, which may cause severe flu-like symptoms that can last for weeks or months.
Vaccination can be carried out with the proven and effective vaccine Leptavoid®-H. This is
the only leptospirosis vaccine that is developed from a UK isolate, is licensed to protect
against both UK strains, and is licensed to improve fertility, where leptospirosis is the cause.
For added convenience and time-saving, Leptavoid®-H can also be given at the same time
as Bovilis® BVD, the UK and Europe’s leading BVD vaccine5.
Farmers are recommended to contact their veterinary surgeon for advice on how to protect
against leptospirosis.
-
Ends -
Image caption: Co-grazing of cattle with sheep and access to water courses are key
leptospirosis risk factors that dairy and beef farmers should be aware of, as well as buying in
and using a bull.
Use medicines responsibly. For more information visit: www.noah.co.uk/responsible
BOVILIS® BVD is an inactivated vaccine containing 50 ELISA units (EU) and inducing at least 4.6 log2 VN
units per dose of cytopathogenic BVD virus strain C86. Legal category: POM-V. BOVILIS BVD is only
available from your veterinary surgeon from whom advice should be sought. BOVILIS BVD is the
property of Intervet International B.V. or affiliated companies or licensors and are protected by
copyrights, trademark and other intellectual property laws. Copyright © 2014 Intervet International B.V. All
rights reserved.
For information regarding side effects, precautions, warnings and contra-indications please refer to the
datasheet at www.noahcompendium.co.uk. Further information is available from MSD Animal Health,
Walton Manor, Walton, Milton Keynes, MK7 7AJ. Tel: 01908 685 685 • vet-support-uk@merck.com •
www.msd-animal-health.co.uk
References:
1 – Expertis™ BeefCheck and DairyCheck data, October 2013 – March 2015
2 – Milk drop due to leptospirosis in dairy cows, Veterinary Record, 7 March 2015, p247-250
3 – Ellis, W.A. et al. (1988), Res Vet Sci, 44, p375-379
4 – UK Zoonoses Report, available at: https://www.gov.uk/government/collections/zoonosesreports
5 - GfK and Ceesa data, MAT February 2015
* Safety and efficacy data are available which demonstrate that this vaccine can be
administered to animals of 8 month of age or older on the same day, with Bovilis
BVD vaccine. The two vaccines should be administered at separate sites. For the
concurrent use of Leptavoid®-H and Bovilis® BVD vaccines in naïve animals, the
primary vaccination course must be completed at least 4 weeks before the expected
gestation, in order that foetal protection can be established.
Leptavoid-H is a vaccine containing Leptospira interrogans serovar hardjo 204 (inactivated). Legal category: POMVPS. Contact your veterinary surgeon or animal health professional for further information and advice. Bovilis BVD is
a vaccine containing BVD virus strain C86. Legal category: POM-V. Bovilis BVD is only available via your veterinary
surgeon from whom advice should be sought. Leptavoid-H and Bovilis BVD are the property of Intervet International
B.V. or affiliated companies or licensors and are protected by copyrights, trademark and other intellectual property
laws. Copyright © 2013 Intervet International B.V. All rights reserved. Further information is available from: MSD
Animal Health Walton Manor Walton Milton Keynes MK7 7AJ Tel: 01908 685685 Fax: 01908 685555 Email: vetsupport.uk@merck.com www.msd-animal-health.co.uk.
Use medicines responsibly. For more information visit: www.noah.co.uk/responsible
BOVILIS® BVD is an inactivated vaccine containing 50 ELISA units (EU) and inducing at least 4.6 log2 VN
units per dose of cytopathogenic BVD virus strain C86. Legal category: POM-V. BOVILIS BVD is only
available from your veterinary surgeon from whom advice should be sought. BOVILIS BVD is the
property of Intervet International B.V. or affiliated companies or licensors and are protected by
copyrights, trademark and other intellectual property laws. Copyright © 2014 Intervet International B.V. All
rights reserved.
For information regarding side effects, precautions, warnings and contra-indications please refer to the
datasheet at www.noahcompendium.co.uk. Further information is available from MSD Animal Health,
Walton Manor, Walton, Milton Keynes, MK7 7AJ. Tel: 01908 685 685 • vet-support-uk@merck.com •
www.msd-animal-health.co.uk
About MSD Animal Health
Today's MSD is a global healthcare leader working to help the world be well. MSD
Animal Health, known as Merck Animal Health in the United States and Canada, is the
global animal health business unit of MSD. Through its commitment to the Science of
Healthier Animals™, MSD Animal Health offers veterinarians, farmers, pet owners and
governments one of the widest range of veterinary pharmaceuticals, vaccines and health
management solutions and services. MSD Animal Health is dedicated to preserving and
improving the health, well-being and performance of animals. It invests extensively in
dynamic and comprehensive R&D resources and a modern, global supply chain. MSD
Animal Health is present in more than 50 countries, while its products are available in some
150 markets. For more information, visit www.msd-animal-health.com or connect with us on
LinkedIn.
MSD forward-Looking Statement
This presentation includes “forward-looking statements” within the meaning of the
safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995.
These statements are based upon the current beliefs and expectations of MSD’s
management and are subject to significant risks and uncertainties. There can be no
guarantees with respect to pipeline products that the products will receive the necessary
regulatory approvals or that they will prove to be commercially successful. If underlying
assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ
materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and
competition; general economic factors, including interest rate and currency exchange rate
fluctuations; the impact of pharmaceutical industry regulation and health care legislation in
the United States and internationally; global trends toward health care cost containment;
Use medicines responsibly. For more information visit: www.noah.co.uk/responsible
BOVILIS® BVD is an inactivated vaccine containing 50 ELISA units (EU) and inducing at least 4.6 log2 VN
units per dose of cytopathogenic BVD virus strain C86. Legal category: POM-V. BOVILIS BVD is only
available from your veterinary surgeon from whom advice should be sought. BOVILIS BVD is the
property of Intervet International B.V. or affiliated companies or licensors and are protected by
copyrights, trademark and other intellectual property laws. Copyright © 2014 Intervet International B.V. All
rights reserved.
For information regarding side effects, precautions, warnings and contra-indications please refer to the
datasheet at www.noahcompendium.co.uk. Further information is available from MSD Animal Health,
Walton Manor, Walton, Milton Keynes, MK7 7AJ. Tel: 01908 685 685 • vet-support-uk@merck.com •
www.msd-animal-health.co.uk
technological advances, new products and patents attained by competitors; challenges
inherent in new product development, including obtaining regulatory approval; MSD’s ability
to accurately predict future market conditions; manufacturing difficulties or delays; financial
instability of international economies and sovereign risk; dependence on the effectiveness of
MSD’s patents and other protections for innovative products; and the exposure to litigation,
including patent litigation, and/or regulatory actions.
MSD undertakes no obligation to publicly update any forward-looking statement,
whether as a result of new information, future events or otherwise. Additional factors that
could cause results to differ materially from those described in the forward-looking
statements can be found in MSD’s 2014 Annual Report on Form 10-K and the company’s
other filings with the Securities and Exchange Commission (SEC) available at the SEC’s
Internet site (www.sec.gov).
###
Use medicines responsibly. For more information visit: www.noah.co.uk/responsible
BOVILIS® BVD is an inactivated vaccine containing 50 ELISA units (EU) and inducing at least 4.6 log2 VN
units per dose of cytopathogenic BVD virus strain C86. Legal category: POM-V. BOVILIS BVD is only
available from your veterinary surgeon from whom advice should be sought. BOVILIS BVD is the
property of Intervet International B.V. or affiliated companies or licensors and are protected by
copyrights, trademark and other intellectual property laws. Copyright © 2014 Intervet International B.V. All
rights reserved.
For information regarding side effects, precautions, warnings and contra-indications please refer to the
datasheet at www.noahcompendium.co.uk. Further information is available from MSD Animal Health,
Walton Manor, Walton, Milton Keynes, MK7 7AJ. Tel: 01908 685 685 • vet-support-uk@merck.com •
www.msd-animal-health.co.uk