Ch_3
advertisement

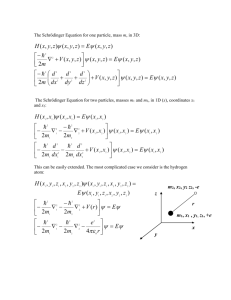
Winter 2013 Chem 356: Introductory Quantum Mechanics Chapter 3 – Schrodinger Equation, Particle in a Box .................................................................................. 34 Introduction to the Schrodinger Equation .............................................................................................. 34 Linear Operators ..................................................................................................................................... 36 Quantization of energy ........................................................................................................................... 39 Interpretation of Wave Function ............................................................................................................ 40 Determination of Constant C .................................................................................................................. 41 Useful integrals for particle in the box ................................................................................................... 43 Demonstration of Uncertainty Principle ................................................................................................. 44 Particle in a 3 dimensional box ............................................................................................................... 46 Chapter 3 – Schrodinger Equation, Particle in a Box Introduction to the Schrodinger Equation De Broglie suggested one can associate a wave with a particle and take p e ikx 2 k p h h k k 2 Generalization to 3 dimensional wave eikx p k In chapter 2 we saw that waves in general satisfy a wave equation. Try to postulate a wave equation for “electron-waves” (a guess) Provide some rational for Schrodinger equation: Wave equation 2u 1 2u x 2 V 2 t 2 Choose solution with particular 2 u ( x, t ) ( x) cos(t ) 34 Chem 356: Introductory Quantum Mechanics Winter 2013 d 2 2 ( x) 0 dx 2 V 2 V 2 v (nu) frequency ; V velocity d 2 4 2 2 ( x) 0 dx 2 4 2 h p 2 2 4 2 p 2 p2 h 2 2 p 2 ( x) 0 dx 2 Now substitute p 2 : p2 V E 2m 2 p 2 ( x) 0 2m x 2 2m 2 2 ( E V ) ( x) 0 2m x 2 2 2 E ( x) V ( x) ( x) 2m x 2 2 Or Hˆ ( x ) We obtain a differential equation for function ( x ) Hˆ ( x) E ( x) E is a constant, the energy Ĥ is “operator” that acts on a function. Summarizing: 2 (using de Broglie + classical wave equation) x 2 2) Substitute p 2 2m( E V ( x)) 1) p 2 ( x) 2 2 V ( x) ( x) E ( x) 2m x 2 Hˆ ( x) E ( x) 2 p2 Hˆ V ( x) 2m ‘energy operator’ (see later) Chapter 3 – Schrodinger Equation, Particle in a Box 35 Winter 2013 Chem 356: Introductory Quantum Mechanics We need to discuss 2 mathematical items p̂ , Ĥ , p̂ 2 ….? a) Operators b) Eigenvalue equations Ĥ E E , p : numbers p̂ p Operators will be indicated by “^” hat or carot Linear Operators (in 1 dimension first) ˆ ( x) g ( x) Af Acting with an operator on a function yields a new function. ˆ ( x) g ( x) Af f ( x)  d2 dx 2 d2 d 2 2 3 dx dx d x dx d x dx d i dx d2 V ( x) 2 2m dx 2x 0 x3 6 x 6 x 2 3x3 x2 2 x2 x d 2 (x ) dx x2 d 2 ( x ) 3x 2 dx e ikx keikx cos(kx) 2k 2 V ( x) cos(kx) 2m The operators we consider are linear operators: Aˆ (c1 f1 ( x) c2 f 2 ( x)) ˆ ( x) c Af ˆ ( x) = c1 Af 1 2 2 Where c1 , c2 are (complex) constants Chapter 3 – Schrodinger Equation, Particle in a Box 36 Winter 2013 Chem 356: Introductory Quantum Mechanics ( f ( x))2 Example of operator that is not linear: SQR( f ( x)) SQR( f ( x) g ( x)) ( f ( x)) 2 ( g ( x)) 2 2 f ( x) g ( x) =SQR( f ( x)) SQR( g ( x)) 2 f ( x) g ( x) Not linear therefore We can act with operators in sequence ˆ ˆ ( x) Aˆ ( Bf ˆ ( x)) ABf In general: ˆ ˆ ( x) BAf ˆ ˆ ( x) ABf d  x , Bˆ dx df d x f ( x) x dx dx d df d ( xf ( x)) f ( x) x x f ( x) dx dx dx Example ˆ ˆ ( x) BAf ˆ ˆ ( x) , for any f ( x ) we write If ABf ˆ ˆ BA ˆˆ 0 AB [ Aˆ , Bˆ ] 0  and B̂ commute, the order does not matter Eigenvalue equations (by example) Aˆ ( x) a ( x) Acting with  on a function yields the same function multiplied by a constant i ikx e (i )(ik )eikx x keikx h 2 ikx e 2 e We say ikx h e i eikx peikx 2 x periodic with period x px eikx pˆ x i pˆ x eikx Number Chapter 3 – Schrodinger Equation, Particle in a Box 37 Winter 2013 Chem 356: Introductory Quantum Mechanics The wave function e ikx is an eigenfunction of operator pˆ x d , with eigenvalue dx pˆ x i k h pˆ ( x) p ( x) A particle with definite momentum px is described by eigenfunction of operator pˆ x Consider kinetic energy operator i 2 2 pˆ d2 x 2m 2m 2m dx 2 2 Eigenfunctions of Kinetic energy: 2 2 2 d 2 ax a e 2 2m dx 2m 0 !! (if areal) Not physical 2 2 2 d sin(ax) + a 2 sin(ax) 2 2m dx 2m Constant Eigenvalue Or 2 2 d2 cos(ax) a 2 cos(ax) 2 2m dx 2m Also 2 eiax 2m a 2 eiax Or Hamiltonian operator: 2 pˆ 2 2 ˆ H V ( x) = V ( x) 2m 2m x 2 Hˆ ( x) E ( x) : particle described by eigenfunction ( x ) has the definite energy E Chapter 3 – Schrodinger Equation, Particle in a Box 38 Winter 2013 Chem 356: Introductory Quantum Mechanics Quantization of energy We saw that a fundamental feature of ‘new’ quantum mechanics was that energy cannot take on any value, but only certain values. Why is that? Let us consider a particle in a box problem: V ( x) 0 0 xa V ( x) elsewhere We wish to solve 2 d2 ( x) V ( x) ( x) E ( x) 2m dx 2 Constant Outside the box V ( x ) we want finite values of E , the only possibility is ( x) 0 outside the box. We also wish ( x ) to be continuous: Inside the box we have V 0 d 2 E ( x) 2m dx 2 2 Boundary Condition: (0) (a) 0 We considered before this equation General Solution: x0 c sin(kx) b cos(kx) 0 b0 E 2 k2 2m Chapter 3 – Schrodinger Equation, Particle in a Box 39 Winter 2013 xa Chem 356: Introductory Quantum Mechanics n , n 1, 2,3 a k c sin(ka) 0 Any c , c not equal to 0 n x a ( x) c sin n 2 2 h 2 n 2 E 2ma 8ma 2 - n 1, 2,3..... Quantization: Combination of wave equation + Boundary conditions n 1, 2, 3 also possible, but yields “same” solutions n x n x c sin c sin a a - c can be anything (still) ˆ ( x) cAˆ ( x) Ac ca ( x) a (c ( x)) If ( x ) is an eigenfunction of operator  then also c ( x ) is eigenfunction. ( c is constant) Interpretation of Wave Function In Mathchapter B we discussed probability distribution p ( x ) dx : p( x) 0 x p( x)dx 1 x xp( x)dx etc. The absolute square of the wave function ( x) * ( x) ( x) is to be interpreted like a probability 2 distribution. p( x)dx ( x) dx 2 Probability to find particle between x and x dx ( x) f ( x) ig ( x) complex f ( x ) , g ( x ) real Chapter 3 – Schrodinger Equation, Particle in a Box 40 Winter 2013 Chem 356: Introductory Quantum Mechanics * ( x) f ( x) ig ( x) * ( x) ( x) [ f ( x) ig ( x)][ f ( x) ig ( x)] f ( x) g ( x) i[ f ( x) g ( x) g ( x) f ( x)] 2 So 2 ( x) 0 2 everywhere Probability distribution ( x) Moreover: 2 dx 1 Normalization Multiply ( x ) by constant c , choose c such that c ( x) new ( x) is normalized Particle in the box (later) 2 n x sin a a n ( x) Further Interpretation xhigh ( x) ( x)dx * xlow Probability to find particle between xlow and xhigh And x x ( x)* ( x)dx Determination of Constant C We will impose that the wave functions are normalized * ( x) ( x)dx 1 For reasons discussed before * ( x) : complex conjugate of functions ( x) f ( x) ig ( x) f ( x ) , g ( x ) real ( x) f ( x) ig ( x) * ( x) ( x) [ f ( x) ig ( x)][ f ( x) ig ( x)] * [ f ( x)]2 [ g ( x)]2 i[ f ( x) g ( x) g ( x) f ( x)] f ( x) g ( x) 2 2 Chapter 3 – Schrodinger Equation, Particle in a Box 41 Winter 2013 Chem 356: Introductory Quantum Mechanics ( x) 0 everywhere 2 If ( x ) is real then ( x) ( x) 2 2 Consider particle in the box wave functions: n ( x) n x a Cn sin 0 xa 0 2 n x ( x) dx 0 Cn sin a dx a 2 2 Cn 2 a 1 2 2 i e a Choose Cn 2 ik e would work too. a We can always choose the function ( x ) to be normalized Simplest, A physically meaningful wave function would be normalized If Aˆ ( x) a ( x) eigenfunction of  , eigenvalue a * ( x) Aˆ ( x) Then * ( x)a ( x) a * ( x) ( x) And: * ( x) Aˆ ( x) dx a * ( x) ( x)dx a 1 IF ( x ) is normalized We define: Aˆ * ( x)Aˆ ( x)dx Called the expectation value of operation  , depending on ( x ) , also called the average value of  If ( x ) is normalized, then  would be the average value measured for quantity A If ( x ) is an eigenfunction of  , then one would always measure a , and the average value A a IF ( x ) is normalized Chapter 3 – Schrodinger Equation, Particle in a Box 42 Winter 2013 Chem 356: Introductory Quantum Mechanics If ( x ) is not an eigenfunction of  , then many values could be obtained if A is measured. The average value would be  One more definition: Aˆ Aˆ 2 : The standard deviation from the average. The spread of the measured values Aˆ Aˆ Aˆ Aˆ Aˆ 2 2 Aˆ Aˆ Aˆ 2 Aˆ 2 2 Aˆ Aˆ Aˆ Aˆ 2 Aˆ 2 2 Depends on wave function ( x ) A2 Useful integrals for particle in the box sin 2 axdx x sin 2ax 2 4a 2 x sin axdx x 2 x sin 2ax cos 2ax 4 4a 8a 2 2 2 x sin axdx x3 x 2 1 cos 2ax 3 sin 2ax x 6 4a 8a 4a 2 Definite Integrals (Most important) a sin 0 a 2 n x a dx a 2 2 x sin 0 a n x a2 dx a 4 2 2 x sin 0 a sin 0 n x a3 a3 dx 2 2 a 6 4n n x n x cos dx 0 a a Chapter 3 – Schrodinger Equation, Particle in a Box 43 Chem 356: Introductory Quantum Mechanics Winter 2013 Demonstration of Uncertainty Principle Using the above integrals, we can calculate the following a) Normalize n Cn sin n x a n x 2 a Cn sin 1 dx Cn a 2 0 2 a 2 Normalized particle in the box eigen states: b) 2 a Cn C 2 n x sin a a Calculate x for normalized n ( x) : 2 n x n x x sin x sin dx a0 a a a 2 a2 a a 4 2 center of the box c) Calculate x 2 2 n x 2 n x sin x sin dx a0 a a a x2 2 a3 a3 2 2 a 6 4n a2 a2 2 2 3 2n d) Standard deviation in x : x 2 x2 x 2 2 2 a2 a2 a2 a2 a a n 2 2 2 2 2 3 2n 12 2n 2 2 n 3 2 2 2 n x d n x sin dx i sin a0 a dx a a e) Px 2 n n x n x cos dx 0 i sin a a 0 a a a f) Px 2 a 2 n x sin a0 a 2 a d 2 n x sin a dx 2 n 2 2 n x 2 sin dx a a 0 a 2 2 2 a 2 Chapter 3 – Schrodinger Equation, Particle in a Box 44 Chem 356: Introductory Quantum Mechanics Winter 2013 n 2 2 h 2 n 2 a2 4a 2 hn ( Px ) 2a 2 ( 2mEn , of course!) We can test the Heisenberg Uncertainty Principle 1 2 n2 2 hn x p 2 2 n 3 2a a 1 2 n2 2 2 2 3 Note 1: x a 12 2 as n is the same as uncertainty in uniform distribution: 1 x2 x a 2 a 0 a 2 a x 2 x Px 11 3 1 x a2 a3 0 3 uniform a2 a2 a2 3 4 12 grows with n. Why? Pn (2mEn ) n 2 2 a2 2 This Spiked distribution Large Uncertainty represents the classical limit of particle of energy En bouncing back and forth in the box Note 2: be calculated for any wave function ( x) Cx(a x) for example: x , x 2 , Px , Px 2 Can also satisfies the boundary conditions Chapter 3 – Schrodinger Equation, Particle in a Box 45 Winter 2013 Chem 356: Introductory Quantum Mechanics Particle in a 3 dimensional box Consider rectangular box of length a, b, c 3D Schrodinger Equation: 2 2 2 ( x, y, z ) E ( x, y, z ) 2m dx 2 dy 2 dz 2 2 Boundary Conditions: (0, y, z ) (a, y, z ) 0 ( x, 0, z ) ( x, b, z ) 0 ( x, y, 0) ( x, y, c) 0 y, z x, z x , y “The wave function at the faces of sides of a box is zero” Technique to solve: Separation of variables. ( x, y, z ) X ( x)Y ( x) Z ( z ) Try Substitute in Schrodinger equation and divide by ( x, y, z ) (as we did for vibrating strings) 2 2 2 1 d2X 1 d 2Y 1 d 2Z E 2m X ( x) dx 2 2m Y ( y ) dy 2 2m Z ( z ) dz 2 This can only be true if each term itself is constant: E x , E y , E z We get 3 equations a) 2 d2X Ex X ( x ) 2m dx 2 2 d 2Y EyY ( y) b) 2m dy 2 c) 2 d 2Z Ez Z ( z ) 2m dz 2 Ex E y Ez E X (0) X (a) 0 Y (0) Y (b) 0 Z (0) Z (c) 0 Chapter 3 – Schrodinger Equation, Particle in a Box 46 Winter 2013 Chem 356: Introductory Quantum Mechanics This is just 3 times the 1D particle in the box equation! We know the (normalized) solution: X ( x) 2 k x sin a a Ex h2 k 2 8m a 2 Y ( y) 2 l y sin b b Ey h2 l 2 8m b2 Z ( z) 2 n z sin c c Ey h2 n2 8m c 2 Or n n n x y z 8 n sin x x abc a ny y sin b 2 nz 2 h 2 nx 2 n y E 2 2 8m a 2 b c nz z sin c nx , ny , nz 1, 2,3.... Degeneracies for Cubic box Consider the special case of a Cubic box a b c . Then the energy takes the form h2 E n 2 n y 2 nz 2 2 x 8ma For each triplet nx , n y , nz we get a different wave function, but different values of nx , n y , nz may yield the same energy. Such energy levels are called degenerate. Eg.for atoms we know there are 1 s-orbital, 3 p-orbitals, 5 dorbitals. Table of energies E h2 8ma 2 14 12 11 9 6 3 n , n , n Degeneracy (1, 2,3), (1,3, 2), (2,1,3), (2,3,1), (3,1, 2), (3, 2,1) (2, 2, 2) (1,1,3), (1,3,1), (3,1,1) (2, 2,1), (2,1, 2)(1, 2, 2) (1,1, 2), (1, 2,1), (2,1,1) (1,1,1) 6 1 3 3 3 1 x y z Chapter 3 – Schrodinger Equation, Particle in a Box 47