ExponentialDecay
advertisement

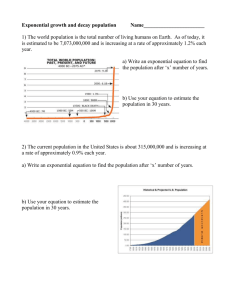
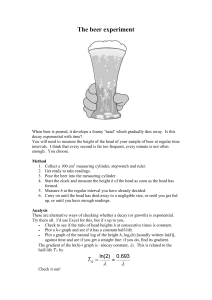
Exponential Decay Claudia Neuhauser CHEM 1231 Fall 2009 Exponential Decay Propylene glycol monomethyl ether (PGME) is a solvent used in surface coatings or cleaners. A study by Domoradzki et al. 2003 examined how quickly PGEM was eliminated from blood in vitro. Doses of 10 or 100 mg/kg were injected into the blood of rats and the concentration of PGME in the blood was monitored. The following table lists the concentration of PGME in μg per g blood at successive time points for the two initial concentrations (low and high). Time [min] 2 10 15 30 45 60 120 240 Concentration PGME [μg/g blood] low high 14.3 10.6 8.2 4.9 2.9 1.3 136.1 113.2 101.6 91.2 91.2 74.8 45.7 10.6 In-class Activity 1 The data is available in the accompanying Excel spreadsheet under tab PGME data. Plot the data in Excel as a Scatter Plot. Transform the axes until the data for each experiment lie along a straight line. Use Trendline to fit an appropriate function. Display the equation of the function and the R2 value for each of the two graphs. Exponential Decay In In-class Activity 1, you found that the best fit was an exponential decay function of the form (1) y(t ) y(0)e kt We will investigate this function in the following. Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 1 Exponential Decay Claudia Neuhauser CHEM 1231 Fall 2009 Successive Ratios Suppose we measured y every h units of time, that is, we would obtain the data y(0), y(h), y(2h), y(3h),... . If we calculated successive ratios y(h) y(2h) y(3h) , , , y(0) y(h) y(2h) We would find that all ratios are identical, namely y(h) kh e y(0) In-class Activity 2 In the spreadsheet under tab Ratio, values of the function y(t ) y(0)e kt at different times are calculated (Column A and B). In Column C, we list successive ratios. (a) Confirm that the value of the ratio does not depend on time t and the value of y(0) , but depends on the parameter k and the time step h. (b) For y(0) 10 , determine the value of the ratio for the following combinations of h and k: h 0.1 0.1 0.2 0.2 k 2 4 2 4 (c) Define the quantity ph as y(t h) 1 ph y(t ) Interpret the quantity ph . Average Velocity The average velocity of a quantity is defined as the ratio of the amount of change during a time interval and the length of the time interval average velocity y(t h) y(t) h Sometimes, it is useful to scale this ratio relative to the current amount of the quantity. We call this new quantity then relative average velocity Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 2 Exponential Decay Claudia Neuhauser (2) CHEM 1231 Fall 2009 relative average velocity 1 y(t h) y(t ) y(t ) h In-class Activity 3 In the spreadsheet under tab RAV, calculate the relative average velocity for h 100,10,1,0.1,0.01,0.001,0.0001 when (a) y(0) 10, k 2 , (b) y(0) 10, k 3 , (c) y(0) 100, k 2 , and (d) y(0) 100, k 3 . Enter the results in the table provided and graph the relative average velocity as a function of h for each of the four cases (a)-(d) above. As h approaches 0, what value does the relative average velocity approach? To answer the question, logarithmically transform the horizontal axis where h is displayed. A Differential Equation Describing Exponential Decay In In-class Activity 3, you found that the relative average velocity (3) 1 y(t h) y(t ) k as h approaches 0 y(t ) h The change in y-values, y(t h) y(t) , is often denoted by y , where the Greek letter Delta, , refers to difference. The quantity h is the time difference of the times when the two data points y(t) and y(t h) were collected and is often denoted by t . We can then write for the relative average velocity relative average velocity 1 y(t h) y(t ) 1 y y(t ) h y t As h, or t , approaches 0, the relative average velocity becomes a relative instantaneous velocity, or a 1 dy relative rate of change, which we denote formally as . With the result in (3), we thus find y dt (4) 1 dy k y dt If we multiply both sides by y, we find for the instantaneous velocity, or rate of change, Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 3 Exponential Decay Claudia Neuhauser (5) CHEM 1231 Fall 2009 dy ky dt Equations (4) or (5) are examples of a first-order differential equation. These kinds of equations relate an instantaneous velocity of a quantity to the quantity itself (or to a function of the quantity). They are studied in detail in calculus. In calculus, you will learn ways to figure out what functions satisfy a given differential equation. We will not concern ourselves with learning these methods at this point. There is computer software that can do this task. For instance, if we wanted to find a function y that satisfied Equation (5), we could use the dy WolframAlpha search engine. For this, we need to know first that instead of , the alternative dt notation y is used. We would enter into the WolframAlpha search engine then y (t ) k * y(t ) 0, y(0) 10 and find that y(t ) 10e kt . Here is a screenshot of the result Figure 1: Screenshot of WolframAlpha solving the differential equation dy ky with y(0) 10 . dt We have now related exponential decay of the form y(t ) y(0)e kt to the rate of change of y(t) , namely, dy ky . dt Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 4 Exponential Decay Claudia Neuhauser CHEM 1231 Fall 2009 Half-Life An important quantity describing exponential decay is the half-life of the quantity, denoted by T, which is defined as the amount of time it takes to reduce the quantity to half its amount. Mathematically, this is expressed as 1 y(t T ) y(t ) 2 With y(t ) y(0)e kt , we find 1 y(0)ek(t T ) y(0)ekt 2 This simplifies to e kT 1 2 Solving for T, T ln2 k In-class Activity 4 For the data in In-class Activity 1, find the half-life for each of the two experiments. In-class Activity 5 (Challenge) The quantity ph , defined above, is related to the parameters k and h as follows ph 1 ekh If we interpret this as the probability that a molecule will decay during the time interval of length h, we can build a stochastic model in Excel that reflects this and compare it to deterministic decay, as defined by Equation (1). Develop the simulation in an Excel spreadsheet and graph both the stochastic decay curve and its corresponding deterministic decay curve in the same graph. Since we know that the functional relationship is described by exponential decay, use a semi-log graph. Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 5 Exponential Decay Claudia Neuhauser CHEM 1231 Fall 2009 Additional Application Hydrolysis Consider a chemical reaction where a chemical compound is hydrolyzed R X H2O k R OH H In this reaction, the compound R-X reacts with water. If water is abundant and the amount of water available remains essentially unchanged during the reaction, the reaction can be considered as a “decay” of a compound. If we denote the amount of the compound R-X by [RX], then the rate of change of disappearance of the compound is described by d[RX] k[RX] dt This is of the same form as Equation (5). We thus expect that [RX] [RX]0 e kt where [RX]0 is the amount available at time 0. References J.Y. Domoradzki, K.A. Brzak, and C.M. Thornton. 2003. Hydrolysis kinetics of propylene glycol monomethyl ether acetate in rats in vivo and in rat and human tissue in vitro. Toxicological Sciences 75: 31-39. Citation: Neuhauser, C. Exponential Decay. Created: July 23, 2009 Revisions: Copyright: © 2009 Neuhauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was partially supported by a HHMI Professors grant from the Howard Hughes Medical Institute. Page 6