Effects of Soy Milk Consumption on Estradiol Concentration in
advertisement

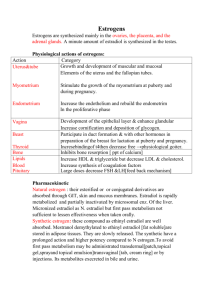
Effects of Soy Milk Consumption on Estradiol Concentration in Saddleback College Males Daniel Mosallaei and Andrew Gomez Department of Biological Sciences Saddleback College Mission Viejo, CA 92692 A variety of plants contain phytoestrogens: compounds that are similar in molecular structure to estradiol, an abundant estrogen, allowing them to exhibit estrogen-like activity. Isoflavones, the primary phytoestrogen in soy, have the potential to cause similar effects upon ingestion. Therefore, an increase in estradiol levels in males should result after a daily consumption of soy milk. Nine Saddleback College males between the ages of 18-25 were chosen for this study. An initial salivary sample was taken from the subjects; each was given a total of four quarts of soy milk to drink over a two week period. Two more samples per subject were collected at the end of the first and second week (Week 1&2) of ingestion. Saliva was collected in 2-mL cryovials and kept in cold storage at -4oC. Once all samples were obtained, they were thawed, centrifuged, and decanted. The samples were analyzed according to Salimetrics EIAEstradiol protocol, and converted to optical density quantities with a micro-plate reader. The microplate outputted optical density values, which were converted to estradiol concentrations using a 4-Paramater Sigmoid Minus Curve. Although an increasing trend was observed, the mean concentrations of the Initial Week (1.66 ±0.388 pg/mL), Week 1 (2.50 ±0.277 pg/mL), and Week 2 (3.45 ±1.05 pg/mL) samples are not statistically different from one another (p =0.183, ANOVA statistical test). Thus, there is no statistical increase in estradiol concentrations in males from drinking four quarts of soy milk over a two week period. Introduction Estrogens are an important group of sex hormones produced in the human body that are associated with development and functioning. Production of these hormones in the body takes place in the testes (males) and the ovaries (females). There are three main estrogenic steroid hormones present in humans: estrone, estriol and, estradiol.; estradiol is the most active estrogen and functions in the body to aid with the sexual reproduction and reproductive development. In addition to its role in sexual and reproductive functioning, estradiol also affects other parts of the body, including the cardiovascular system, the brain, and the immune system in both males and females (McCarthy, 2008; Balthazart, 2006). Estrogenic hormones are present in both men and women however; estrogen levels are significantly higher in women. Phytoestrogens are plant-derived compounds (phyto=plant) from a wide variety of plants that have similar chemical structures as estrogenic hormones. Soy contains isoflavones, a type of phytoestrogen, which are said to exhibit estrogen-like activity and, more recently, have been reported to display both estrogenic and antiestrogenic effects (Davis, 1999). Soy consumption has been suggested to exert potentially cancerpreventive effects in premenopausal women, such as increased menstrual cycle length and sex hormone-binding globulin levels and decreased estrogen levels. There has been some concern that consumption of phytoestrogens might exert adverse effects on men’s fertility, such as lowered testosterone levels and semen quality (Kurzer, 2002). This study investigated if there would be an increase in estradiol levels of Saddleback males after a daily consumption of soymilk over a twoweek period. Materials and Methods Nine adult males between the ages of 18-25 were used in this study. These subjects were students attending Saddleback College, Mission Viejo, California, enrolled in the Biology 3B course for Fall 2013. All subjects were approached and agreed to participate in the study; a waiver was signed by each. Three saliva extractions were taken between 10:30 a.m. and 12:00 p.m. on 6th, 13th, and 20th November, 2013 using labeled 2mL Salimetrics saliva cryovials. After rinsing their mouths ten minutes beforehand, the subjects used the provided saliva collection aid (SCA) to deliver about 1.5 mL (3/4ths full) saliva to the cryovials. The cryovials were labeled with the week number and the name of the participant, and after they were placed in a -4oC freezer to be held until analysis. After the first extraction (Initial Sample) on the 6th November, 2013, participants commenced the drinking of the daily soy milk. Each day, subjects ingested one cup of soy milk at the same time each day for the remaining the two weeks. Throughout the course of this two week drinking period, multiple emails were sent to participants as reminders to continue drinking the milk. Otherwise, the subjects made no changes to the rest of their diet – as advised. At the end of each week during the soy milk ingestion, a second and third saliva sample was collected from each subject (Week 1 & 2 Samples). Aforementioned, the salivary extractions were frozen at -4oC until all samples were collected to be analyzed. Once the three sets of samples were obtained on the 20th November, 2013, they were taken out of cold storage and thawed. The bags holding the cryovials were placed in warm areas to increase the rate of melting. After, the samples were transferred to smaller tubes and stirred using a micro centrifuge, separating the solid and liquid in the bacteria. The supernatant liquid was decanted from the salivary precipitate. An analysis – using Salimetrics Enzyme Immunoassay (EIA) kit reagents – was performed to quantify the concentration of estrogen within each supernatant sample. The wells on the microplate were mapped out for each subject and their respective saliva samples. The experimenters used the kit to fully analyze the samples according to Salimetrics Salivary 17β-Estradiol EIA Protocol. The microtitre plate is already coated with estradiol antibodies, to which the estradiol from the saliva and the standards compete with the estradiolperoxidase for a spot on the antibodies. Therefore, the greater the estradiol concentration in the sample, the less estradiol-peroxidase will be able to bind to the coated antibodies. The tetramethylbenzidine (TMB) combined with the stop solution (2M sulfuric acid) detects the amount of estradiolperoxidase and exerts a level of opaqueness, or optical density. When this optical density becomes available, the micro-plate was read by a Fischer Scientific Multiskan micro-plate reader at 450 ƞm, giving assorted quantitative data of optic density for each well. Since the value outputted by the microplate reader is proportional to the amount of estradiol-peroxidase, this value will be the inverse of the actual estradiol concentration. These values were transferred to MS Excel (Microsoft Corporation, Redmond, Washington) where further statistical manipulations and processing were performed. The sample and standard values were inputted into a MS Excel Estradiol spreadsheet created by Yousef Azzazy. Based on the standard estradiol concentrations, the spreadsheet produced a fitted 4-Parameter Sigmoid Curve embedding the samples’ concentrations. To output the most accurate data, the experimenters adjusted the four parameters of the Sigmoid Curve to obtain a best−.59 fit: 𝐹(𝑥) = 0.7 + 𝑥 −1.45 . Aforementioned, the 1+( ) 4 actual estradiol levels will be the inverse of the data outputted by the microplate; therefore, the inverse 4-parameter sigmoid curve was used to obtain the concentrations. These values – containing three estradiol concentrations for each of the nine subjects – were transferred into another MS Excel spreadsheet. The mean estradiol concentration of each week was obtained, and compared using an ANOVA test. The differences in concentration between the three weeks was also compared and analyzed with a paired T-test. Results As mentioned above, the data was run through Yousef’s excel program to output the 4Parameter Sigmoid Minus Curve. The curve, 𝐴−𝐷 represented by the equation 𝐹(𝑥) = 𝐷 + 𝑥 𝐵 was 1+( ) 𝐶 adjusted to fit along the plot points created by the standards as best as possible. The minimum asymptote (A) was set at 0.11; the steepness (B) was changed to -1.45; the inflection point (C) fit the scatterplot best at 4, and the maximum asymptote (D) was set to 0.7. This graph is shown in Figure One. The original function created by the sigmoid curve outputs the log of concentration; therefore, to obtain the estradiol concentrations the inverse of the function was taken. Based on the inverse sigmoid function, the lower the ratio of optical density of Bound (B) to Unbound (Bo) estradiol-peroxidase of the saliva sample, the higher the estradiol concentration. Aforementioned, this is because the concentration of estradiol is inversely proportional to the level of estradiol-peroxidase. The mean estradiol concentrations for Initial, Week 1, and Week 2 samples were calculated. For the Initial sample, the average estradiol concentration was 1.66 ± 0.388 pg/mL (± SEM, C.L. ±0.894, N=9). At the end of Week 1, the mean concentration was calculated to be 2.50 ± 0.277 pg/mL (± SEM, C.L. ±0.638, N=9). For Week 2, the mean estradiol concentration was 3.45 ± 1.05 pg/mL (±SEM, C.L. ±2.41, N=9). These data are shown in Figure Two. The three mean estradiol concentrations were run through an ANOVA statistical test, revealing p=0.183. Furthermore, the mean increase in estrogen concentrations between the first and second week, the second and third week, and the first and third week were calculated and found to be 0.833 pg/mL, 0.95652 pg/mL, and 1.71 pg/mL respectively. An ANOVA test was also run for these increases, outputting a p value of 0.533. A one-tailed paired equal variance t-test was run to test for statistical difference in the sequential increases between the three weeks, revealing p = 0.4273. Finally, a one-tailed paired equal variance ttest was run between the mean concentrations of the first and final week (Initial and Week 2), outputting p = 0.072. Figure 2. A bar graph comparing the Week the Sample was taken with Estrogen Concentration (n=9). Initial sample mean concentration was 1.66 ± 0.388 pg/mL. Week 1 mean concentration was 2.50 ± 0.277 pg/ mL. Week 2 mean estradiol concentration was 3.45 ± 1.05 pg/mL. There is no statistical difference between the three weeks (? = 0.183, ANOVA statistical test). Error Bars are mean ± SEM. Ratio of optical density (B/Bo) 0.7 0.6 𝐹 𝑥 =𝐷+ 0.5 𝐴−𝐷 𝑥 1 + ( )𝐵 𝐶 0.4 0.3 0.2 0.1 0 1 10 Log of Estradiol Concentration (pg/ml) 100 Figure 1. Four Parameter Sigmoid Minus Curve relating the Percent Bound of Standards and Unknowns with the Log of Testosterone Concentration (n=9). As the log of concentration increases, the ratio of bound to unbound decreases. Estrogen Concentration (pg/mL) 5 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 Initial Mean Week 1 Mean Week 2 Mean Week of Sample Discussion Based off initial observations of Figure Three, an increasing trend can be seen between the mean estradiol concentrations between the three separate samples – which would confirm the hypothesis. However, the statistical tests proved otherwise. When the microplate reader scanned the microplate, it generated a quantitative value based on optical density of each well; these optical densities acted as the raw data. The raw data was converted to estradiol concentration values using the Inverse Sigmoid Minus Curve function that was generated with the high/low concentration standards and four parameters. Even though an increasing trend in estradiol concentrations with soy milk consumption was observed, statistical tests are needed for confirmation. An ANOVA test comparing the means of the three weeks was inconclusive, outputting 0.183. Furthermore, a second ANOVA test was run comparing the differences between each week, which outputted a p value of 0.533 – another statistically insignificant result. Furthermore, a one tailed, paired T-test comparing the linear/sequential increase between the weeks (Initial and Week 1, Week 1 and Week 2) came out undesirable, with a p value of 0.4273. Thus far, the trend observed is not statistically backed up, meaning there is no significant difference between the weeks of soy milk ingestion and the week without it. A final one-tailed paired t-test was run comparing the first week (Initial) – before any soy milk was ingested – and the final week (Week 2) – after two weeks of soy milk was ingested, revealing p = 0.072. Relative to the others, this is the lowest p value; yet since it is greater than 0.05, there is no statistical one-direction difference of the estradiol concentrations between the first week (without soy milk) and final week (after two weeks of soy milk ingestion), rejecting the proposed hypothesis. This study was performed over a three-week period; a continuation of this study could be achieved by performing the experiment over a longer duration of time to examine any greater change in estrogen levels. This would include an increase in the period of time for soymilk ingestion. A study performed by Jorge E. Chavarro in 2008 suggested that a higher intake of soy foods and soy isoflavones had a relation with lower sperm concentration among men. Furthermore, an increased amount of soymilk intake for each subject to consume per week could be observed. Increased soymilk consumption could have a greater effect on the estradiol levels of males in this experiment. R.C. Griggs experimented on the effect of testosterone on body size and concluded that testosterone increases the muscle mass of men by increasing muscle protein synthesis; therefore, body size could have an effect on the amount of soymilk needed for ingestion. Perhaps, individuals of bigger size require higher dosage of soy to obtain a change in estradiol levels. A study done in 1978 by Martin M. Pierre on phytoestrogens reported that phytoestrogens had the ability to interact with estrogen receptors in human breast cancer cells, suggesting that phytoestrogens could potentially inhibit the production of breast cancer cells. Phytoestrogens are still a great area of study for scientists to this day. Literature Cited Balthazart, J., Cornil, C.A., Taziaux, M., et al. (2006). Rapid changes in production and behavioral action of estrogens. Neuroscience, 138(3), 783-91. Chavarro, Jorge E., Toth, Thomas L., Sadio, Sonita M., Hauser, Russ. (2008). Soy food and isoflavones intake in relation to semen quality parameters among men from an infertility clinic. Oxford Journals 23(11), 2584-2590. Davis, SR., Dalais, F.S., Simpson, E.R., Murkies, A.L. (1999). Phytoestrogens in health and disease. Jean Hailes Foundation Research Unit, 210(1), 185-210. Griggs, R.C., Kingston, W., Jozefowicz, R.F., Herr, B.E., Forbes, G., Halliday, D. (1989). Effect of testosterone on muscle mass and muscle protein Martin, Pierre M., Horwitz, Kathryn B., Ryan, Dale S., Mcguire, William L. (1978). Phytoestrogen Interaction with Estrogen receptors in Human Breast Cancer Cells. Endocrinology 103(5), 18601867. McCarthy, M.M. (2008). Estradiol and the Developing Brain. Physiol Rev, 88(1), 91-134. synthesis. Journal of Applied Physiology 66(1), 498-503.