Atomic Emission Spectra of Hydrogen
advertisement

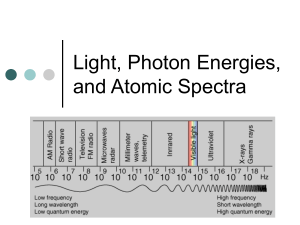
Atomic Emission Spectra of Hydrogen Thomas Ehret Adam Cooke, Marc Hnatyshin B.C. Boivin SPH4U, Grade 12 University Physics, Rockland District High School Friday, April 19th, 2013. Introduction: The goal of this lab is to accurately measure the energy of various light waves for the atomic emission spectra of Hydrogen. The Electromagnetic Spectrum is a scale that shows the correlation between wavelength and frequency of all waves with electromagnetic radiation (See Figure 1.). When the wavelength of the electromagnetic radiation waves is larger, the frequency is lower, and when the wavelength is smaller, the frequency is higher. The radiation of the waves with higher frequencies like Gamma Rays and XFigure 1. The Electromagnetic Spectrum Rays tend to be the most harmful. The demonstrating wavelengths and frequencies (Anonymous, 2012). Visible Spectrum portion of the Electromagnetic Spectrum is the only part of the scale that the human eye can witness. The Atomic Emission Spectra describes the specific frequencies emitted by an atom when it is subject to having energy inputted into it. This was discovered because it was found Figure 3. 2. The Atomic Emission Spectra of Hydrogen. Colours shown from the four distinct frequencies given off (Anonymous, How Our Body Might Collect Information, 2011). that orbits become quantized and allow electrons to jump from orbit to orbit. When the orbit they jump to has more energy than the original, the electron absorbs a photon with a specific frequency, but when it jumps to an orbit with less energy, the electron gives off a photon with a specific frequency (Anonymous, atomic spectrum, 2005). A photon is described to be a quanta of light or in simpler terms, a particle. It is a packet of energy that moves in the form of a wave which makes light unique towards other waves (Anonymous, Photons As Light Quanta). The specific frequencies that these photons are giving off differ for different types of atoms and elements. For example, a Figure 4. Planck’s constant shown in an example where an electron is jumping to another orbit (Norton, 2013). Hydrogen atom will normally give off frequencies that demonstrate the colours red (𝜆 = 656.2nm), teal (𝜆 = 486.1nm), indigo (𝜆 = 434nm), and violet (𝜆 = 410.1nm) that each, as shown, have their own specific wavelength as well (Anonymous, Emission Spectrum of Hydrogen). These colours are in the order that you would see them on the electromagnetic spectrum (See Figure 2.). Niels Bohr was one of the scientists that discovered and theorized about what was happening when electrons jump from orbit to orbit (See beginning of second paragraph). One of which was that there are certain orbits in atoms where the electrons do not emit energy. Another was that when there is an emission or absorption of radiation, it means that an electron is jumping from one orbit to another (Students). Max Planck was a theoretical physicist who helped develop the quantum theory. He discovered that action did not take on any value for different situations. Planck then came up with the constant, ‘quantum of action’ which is now called Planck’s constant. This constant appears in equations used in physics such as: Δ𝐸 = ℎ𝑓, where ‘h’ is the symbol used for the constant (See Figure 3.). The purpose of this experiment was to experimentally determine the energy of each light wave of the atomic emission spectra of Hydrogen by reenacting Bohr’s lab Rockland style. Theory: 𝑐 = 𝜆𝑓 𝑐 - speed of wave, or speed of light (usually speed of light) 𝜆 - wavelength of the wave 𝑓 - frequency of the wave Δ𝐸 = ℎ𝑓 Δ𝐸 - Change in energy of the wave ℎ - Planck’s constant, quantum of action (Anonymous, Planck constant, 2013). 𝑓 - frequency of the wave %𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙| 𝑥 100% 𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 Materials and Methods: The materials and methods used in this experiment were those of the class, SPH4U on Thursday, April 11th, 2013. Figure 5. Atomic Emission Spectra of Hydrogen lab setup. Asif Nazerally looking through a spectroscope into the hydrogen light source (Hendrix-Sicard). Results and Observations: Table 1. Atomic Emission Spectra for 𝐻2 . Experimental values. Spectral Line Colour 𝒇 (Hz) 𝝀 (nm) Red Teal (Blue-Green) Indigo (Blue-Violet) Violet 700 500 475 450 14 4.29 𝑥 10 6 𝑥 1014 6.32 𝑥 1014 6.67 𝑥 1014 𝜟𝑬 (J) 2.84 𝑥 10−19 3.98 𝑥 10−19 4.19 𝑥 10−19 4.42 𝑥 10−19 Table 2. Atomic Emission Spectra for 𝐻2 . Empirical values. Spectral Line Colour 𝒇 (Hz) 𝝀 (nm) Red Teal (Blue-Green) Indigo (Blue-Violet) Violet 656.2 486.1 434 410.1 Calculations: Red (experimental): 𝑐 𝑓= 𝜆 𝑐 = 3 𝑥 108 𝑚/𝑠 3 𝑥 108 𝑚/𝑠 𝑓= 700 𝑥 10−9 𝑚 𝑓 = 4.29 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 ℎ = 6.63 𝑥 10−34 𝐽 𝑥 𝑠 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(4.29 𝑥 1014 𝐻𝑧) Δ𝐸 = 2.84 𝑥 10−19 𝐽 Red (empirical): 𝑐 𝑓= 𝜆 3 𝑥 108 𝑚/𝑠 𝑓= 656.2 𝑥 10−9 𝑚 𝑓 = 4.57 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(4.57 𝑥 1014 𝐻𝑧) 14 4.57 𝑥 10 6.17 𝑥 1014 6.91 𝑥 1014 7.32 𝑥 1014 𝜟𝑬 (J) 3.03 𝑥 10−19 4.09 𝑥 10−19 4.58 𝑥 10−19 4.85 𝑥 10−19 Δ𝐸 = 3.03 𝑥 10−19 𝐽 Red (Percentage of error): %𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙| 𝑥 100% 𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 |3.03 𝑥 10−19 − 2.84 𝑥 10−19 | %𝐸𝑟𝑟𝑜𝑟 = 𝑥 100% 3.03 𝑥 10−19 %𝐸𝑟𝑟𝑜𝑟 = 6.27% Teal (experimental): 𝑐 𝑓= 𝜆 𝑐 = 3 𝑥 108 𝑚/𝑠 𝑓= 3 𝑥 108 𝑚/𝑠 500 𝑥 10−9 𝑚 𝑓 = 6.00 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 ℎ = 6.63 𝑥 10−34 𝐽 𝑥 𝑠 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(6.00 𝑥 1014 𝐻𝑧) Δ𝐸 = 3.98 𝑥 10−19 𝐽 Teal (empirical): 𝑐 𝑓= 𝜆 𝑓= 3 𝑥 108 𝑚/𝑠 486.1 𝑥 10−9 𝑚 𝑓 = 6.17 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(4.57 𝑥 1014 𝐻𝑧) Δ𝐸 = 4.09 𝑥 10−19 𝐽 Teal (Percentage of error): %𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙| 𝑥 100% 𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 |4.09 𝑥 10−19 − 3.98 𝑥 10−19 | %𝐸𝑟𝑟𝑜𝑟 = 𝑥 100% 4.09 𝑥 10−19 %𝐸𝑟𝑟𝑜𝑟 = 2.69% Indigo (experimental): 𝑐 𝑓= 𝜆 𝑐 = 3 𝑥 108 𝑚/𝑠 𝑓= 3 𝑥 108 𝑚/𝑠 475 𝑥 10−9 𝑚 𝑓 = 6.32 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 ℎ = 6.63 𝑥 10−34 𝐽 𝑥 𝑠 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(6.32 𝑥 1014 𝐻𝑧) Δ𝐸 = 4.19 𝑥 10−19 𝐽 Indigo (empirical): 𝑓= 𝑐 𝜆 3 𝑥 108 𝑚/𝑠 𝑓= 434 𝑥 10−9 𝑚 𝑓 = 6.91 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(6.91 𝑥 1014 𝐻𝑧) Δ𝐸 = 4.58 𝑥 10−19 𝐽 Indigo (Percentage of error): %𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙| 𝑥 100% 𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 %𝐸𝑟𝑟𝑜𝑟 = |4.58 𝑥 10−19 − 4.19 𝑥 10−19 | 𝑥 100% 4.58 𝑥 10−19 %𝐸𝑟𝑟𝑜𝑟 = 8.52% Violet (experimental): 𝑐 𝑓= 𝜆 𝑐 = 3 𝑥 108 𝑚/𝑠 𝑓= 3 𝑥 108 𝑚/𝑠 450 𝑥 10−9 𝑚 𝑓 = 6.67 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 ℎ = 6.63 𝑥 10−34 𝐽 𝑥 𝑠 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(6.67 𝑥 1014 𝐻𝑧) Δ𝐸 = 4.42 𝑥 10−19 𝐽 Violet (empirical): 𝑐 𝑓= 𝜆 3 𝑥 108 𝑚/𝑠 𝑓= 410.1 𝑥 10−9 𝑚 𝑓 = 7.32 𝑥 1014 𝐻𝑧 Δ𝐸 = ℎ𝑓 Δ𝐸 = (6.63 𝑥 10−34 𝐽 𝑥 𝑠)(7.32 𝑥 1014 𝐻𝑧) Δ𝐸 = 4.85 𝑥 10−19 𝐽 Violet (Percentage of error): %𝐸𝑟𝑟𝑜𝑟 = |𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 − 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙| 𝑥 100% 𝐸𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 %𝐸𝑟𝑟𝑜𝑟 = |4.85 𝑥 10−19 − 4.42 𝑥 10−19 | 𝑥 100% 4.85 𝑥 10−19 %𝐸𝑟𝑟𝑜𝑟 = 8.87% Discussion: In this experiment, the students used a spectroscope to look at a Hydrogen discharge tube to see the atomic emission spectra of Hydrogen. The atomic emission spectra of Hydrogen will show the colours red, teal, indigo, and violet, and has a set of empirical values for the colours` wavelength, frequency, and energy. The goal was to experimentally get values that were close to the empirical values. The percentage of errors calculated in order by red, teal, indigo, and violet were 6.27%, 2.69%, 8.52%, and 8.87%. All these values are less than 10% which would make it considerably a successful experiment. There are a few possible sources of error that could have skewed the results to make them unequal to the empirical values. One could be that there was too much light in the room when conducting the experiment. Ideally when doing experiments where there is light involved, having a room with very little light is better. The light can degrade the light coming from the discharge tube and generally give you inaccurate results. This could have been fixed by planning the experiment earlier and using the classroom that does not have any windows. Another possible source of error is that the Hydrogen discharge tube being used for the experiment was not pure, meaning that it was not pure Hydrogen emitting the light. Another gas such as Argon or Neon could have been helping with the emission of the light. This would skew the results by giving the wrong colour input into the spectroscope which would give us a skewed wavelength value. This could only be fixed by somehow purifying the discharge tube or buying a new discharge tube that was purely Hydrogen. A third possible source of error could be that the spectroscopes that were used are old and not completely functional. This could skew the results by again, giving a slightly different value for the wavelength causing all your calculations to be off. The only solution for this would be to buy new, perfectly functional spectroscopes. Conclusion: It was experimentally determined that the energy for red was 2.84 𝑥 10−19 𝐽 with a percentage of error of 6.27%, for teal was 3.98 𝑥 10−19 𝐽 with a percentage of error of 2.69%, for indigo was 4.19 𝑥 10−19 𝐽 with a percentage of error of 8.52%, and for violet was 4.42 𝑥 10−19 𝐽 with a percentage of error of 8.87%. Selected References: Anonymous. (2005). atomic spectrum. Retrieved April 16, 2013, from The Free Dictionary: http://www.thefreedictionary.com/Atomic+emission+spectrum Anonymous. (2012, August 5). Dr. Chemical. Retrieved April 16, 2013, from Dr. Chemical: http://drchemical.com.au/category/uncategorized/page/2 Anonymous. (n.d.). Emission Spectrum of Hydrogen. Retrieved April 18, 2013, from Chem-Ed: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/bohr.html Anonymous. (2011, January 26). How Our Body Might Collect Information. Retrieved April 16, 2013, from Touchy Subjects: http://touchysubjects.wordpress.com/2011/01/26/how-our-bodymight-collect-information/ Anonymous. (n.d.). Photons As Light Quanta. Retrieved April 18, 2013, from Michigan State University Physics 232: http://www.pa.msu.edu/~pratts/phy232/lectures/quantum/photons.html Anonymous. (2013, April 4). Planck constant. Retrieved April 17, 2013, from Wikipedia: https://en.wikipedia.org/wiki/Planck_constant Hendrix-Sicard, M. Lab setup . Rockland. Norton, J. D. (2013, April 6). Origins of Quantum Thoery. Retrieved April 18, 2013, from Pitt Education: http://www.pitt.edu/~jdnorton/teaching/HPS_0410/chapters/quantum_theory_origins/ Students. (n.d.). Atomic Spectra and the Bohr's Theory. Retrieved April 18, 2013, from Think Quest: http://library.thinkquest.org/28383/nowe_teksty/htmla/1_12a.html