Ceren Eroglu - Topic 7 Atomic and Nuclear
advertisement

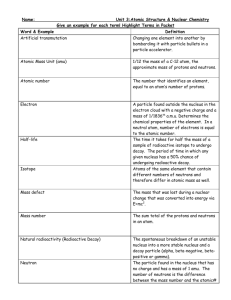
Topic 7: Atomic and nuclear physics 7.1 The atom Atomic structure 7.1.1 Describe a model of the atom that features a small nucleus surrounded by electrons The atom has a positively charged small nucleus and is surrounded by negatively charged electrons orbiting it. 7.1.2 Outline the evidence that supports a nuclear model of the atom The Rutherford (Geiger-Marsden experiment). The experiment consisted of firing very small, fast moving alpha particles at a very thin gold foil. Had the previous model (J. J. Thompson’s Plum Pudding Model) been true, then the alpha particles would have stuck to the gold foil. Instead, some of the alpha particles bounced back, and some even went through. The fact that certain particles went through suggested that there was empty space within the atom; the fact that certain particles bounced back indicated that they had a head-on collision with a heavy, positively charged particle. Rutherford therefore concluded that the atom had a small, positive nucleus, surrounded by negatively charged electrons. *Evidence for the existence of the nucleus. 7.1.3 Outline one limitation of the simple model of the nuclear atom The outer electrons, having centripetal acceleration, should radiate, lose energy and spiral into the nucleus. What actually happens: electrons show quantum behavior that allows them to exist in different energy levels. Radiation is therefore not emitted except when the electrons change energy levels. 7.1.4 Outline evidence for the existence of atomic energy levels Atomic spectra provide evidence that the energy in atoms is discrete (there are energy levels). When white light passes through a certain material (e.g. hydrogen), various wavelengths of light are emitted. These wavelengths correspond to the emission line wavelengths; this is because the electrons absorb the photons and use the energy to change energy levels. Nuclear structure 7.1.5 Explain the terms nuclide, isotope and nucleon Nuclide: a combination of protons and neutrons that form a nucleus. Isotope: nuclei with the same number of protons but different numbers of neutrons. Nucleon: the name given to the particles of the nucleus (proton, neutron). 7.1.6 Define nucleon number A, proton number Z and neutron number N 1 Nucleon number A: the number of protons plus neutrons in the nucleus. This will be different for different isotopes of the same element. Proton number Z: the number of protons in the nucleus. This is always the same for a given element. Neutron number N: the number of neutrons in a nucleus. 7.1.7 Describe the interactions in a nucleus The two forces within the nucleus: 1. The strong nuclear force (between nucleons, mostly attractive, short-range) 2. The weak electrical repulsive force (between protons, repulsive, long-range) 7.2 Radioactive decay Radioactivity 7.2.1 Describe the phenomenon of natural radioactive decay Natural radioactive decay happens when nuclei are unstable and try becoming more stable by decaying (emitting either alpha or beta particle, or gamma rays). 7.2.2 Describe the properties of alpha () and beta () particles and gamma () radiation Alpha Beta Gamma Made of A helium nucleus (2 A loose electron Photons protons, 3 neutrons) Range in air (approx) 5 cm (approx) 30 cm Electromagnetic radiation (doesn’t emit particles, simply loses energy) Stopped by Paper Aluminum Lead 7.2.3 Describe the ionizing properties of alpha () and beta () particles and gamma () radiation Ionization is the process of a photon of high frequency and therefore high energy absorbs another photon and causes an electron to be ejected from an atom. Alpha Beta Gamma Penetration Least Most Ionization Most Least 7.2.4 Outline the biological effects of ionizing radiation Very high dose- affects the central nervous system, loss of coordination and death. Medium dose- damages the stomach and intestines, sickness and diarrhea, potential death. Low dose- loss of hair, bleeding and diarrhea. Long term- cancer or gene mutations in the case of pregnancy. 7.2.5 Explain why some nuclei are stable while others are unstable If the nucleus contains too many protons compared to neutrons, the repulsive force will overpower the attractive force and the nucleus will be unstable. A small stable nucleus has approximately the same number of protons as those of neutrons. A larger nucleus also has approximately the same number of protons as those of neutrons, except the number of neutrons will be several higher since neutrons are the “glue” 2 holding the nucleus together. The greater the difference of the amount of protons and neutrons, the more unstable the nucleus will be. Half-life 7.2.6 State that radioactive decay is a random and spontaneous process and that the rate of decay decreases exponentially with time Decay is random (it cannot be known which nucleus or when decay will happen) and spontaneous (it cannot be known when it is happening or be prevented). Decay decreases exponentially over time. The nature of the decay is independent of the initial amount. 7.2.7 Define the term radioactive half-life The time taken for half of the nuclei in a sample to decay. 7.2.8 Determine the half-life of a nuclide from a decay curve The half-life of a nuclide can be determined by looking at the point at which half of the nuclei remain and finding the time period associated with it, as it is the case with the graphs above. The one on the left shows the half-life of a nuclide, and the one on the right shows several half-lives. 7.2.9 Solve the radioactive decay problems involving integral numbers of halflives The half-life of an isotope is 4.0 days. Calculate the fraction of the original activity of the isotope 12 days after it has been prepared. An isotope X has a half-life of 2.0 minutes. It decays into isotope Y that is stable. Initially no quantity of isotope Y is present. After how much time will the ratio of Y atoms to X atoms be equal to 15? A radioactive isotope has a half-life of 5.0 minutes. A particular nucleus has not decayed within a 5.0 min time interval. A correct statement about the next 5.0 min interval is that this nucleus A has a lower than 50% chance of decaying B will certainly decay. C has a 50% chance of decaying. D has a better than 50% chance of decaying. 7.3 Nuclear reactions, fission and fusion 3 Nuclear reactions 7.3.1 Describe and give an example of an artificial (induced) transmutation Transmutation is when radioactive particles are emitted from the nucleus not because the nucleus is unstable but because another nucleon is added to the original nucleus. For example, the production of nitrogen from carbon is possible through the artificially initiated reaction by the high-energy particles. 7.3.2 Construct and complete nuclear equations This website has exercises on constructing and completing nuclear equations: http://www.chemtopics.com/unit11/ppu11.pdf 7.3.3 Define the term unified atomic mass unit Unit used for masses on atomic or molecular state. 1 u = 1.66x10-27 kg The unified atomic mass unit is 1/12 of the mass of the neutral atom of the carbon isotope carbon-16. 7.3.4 Apply the Einstein mass-energy equivalence relationship E = mc2 If work is done, then there must be some sort of transfer of energy. The energy has been converted to mass. The mass of the particles when they are apart is greater than when they are together. 7.3.5 Define the concepts of mass defect, binding energy and binding energy per nucleon Mass defect: the difference between the mass of the whole nucleus and the mass of the parts of a nucleus. Binding energy: the amount of work required to pull apart the constituents of a nucleus. Binding energy per nucleon: the amount of work required to pull apart the constituents of a nucleus per nucleon. 7.3.6 Draw and annotate a graph showing the variation with nucleon number of the binding energy per nucleon 7.3.7 Solve problems involving mass defect and binding energy 4 This website has a thorough summary of radioactive decay and practice problems (with solutions): http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/modes.php Fission and fusion This is an other website that has more information on fission and fusion, it is a good resource to look through: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/fission.php#fusion 7.3.8 Describe the processes of nuclear fission and nuclear fusion Fission: the nuclear reaction of the nucleus of an atom splitting into two smaller pieces. Fusion: the joining up of two small nuclei to form a big one. Energy is released in both processes. 7.3.9 Apply the graph in 7.3.6 to account for the energy release in the processes of fission and fusion The masses of the individual atoms add up to be more than the mass of the whole, therefore there must be some source of mass/energy loss; therefore in fission, where the nucleus of an atom splits, the energy released is that of the binding energy of the nuclei (holding the atom together). In fusion, where two nuclei combine to form another one, the energy released is that of the parts fusing together; the binding energy as well. The reason fusion releases more energy is because the difference in the binding energies of the elements in fusion is greater than those of fission. 7.3.10 State that nuclear fusion is the main source of the Sun’s energy Nuclear fusion is the main source of the Sun’s energy. 7.3.11 Solve problems involving fission and fusion reactions This is a web link to Giancoli problems on chapter 30. They have hints and cover fission, fusion and some other topics related to nuclear physics. (It also has links to other Giancoli chapters and practice problems) : http://wps.prenhall.com/esm_giancoli_physicsppa_6/17/4362/1116732.cw/index.html 5