Nuclear Marbles LP
advertisement

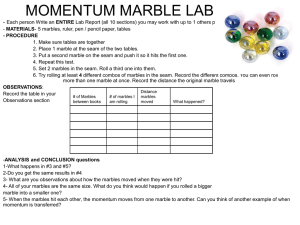
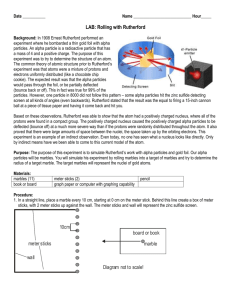
Lesson Idea Template Instructional Alignment to Three Dimensions of SDSS Student Science Performance Grade: HS Chemistry Title Nuclear Marbles Lab Topic : Rutherford's Gold Foil Experiment Standard from South Dakota Science Standards: HS-PS1-2 Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties. Lesson Performance Expectations: Students use representations to reflect on phenomena and describe the structure relationships. Students use mathematical expressions to represent phenomena to support explanations in systems that are too small to observe directly. Students collect data and generate evidence to answer scientific questions to describe the structure relationships. Student Science Performance Gathering (Teacher Tip: Prior to beginning this activity, students should be assigned a reading on Rutherford's Gold Foil Experiment, including basic comprehension questions). Experiment based on http://www.laserpablo.com/teacherresources/files/APLabs_CCPhysics/45-Nuclear_Marbles.pdf Investigate phenomena and describe the structure relationships: Address prior learning by discussing Rutherford's Gold Foil Experiment. Correct any questions or misunderstandings. Using mathematical expressions to represent phenomena to support explanations: Discuss the equation in the lab: where R is the radius of the large marble, r is the radius of the small marble, N is the number of small marbles, L is the width of the target box, H is the number of trials that resulted in at least one hit, and T 𝑝𝑎𝑡ℎ 𝑤𝑖𝑑𝑡ℎ 𝑁(2𝑅+2𝑟) 𝐻 is the total number of trials. 𝑃 = = = (Teacher Tip: Bowling may be useful to describe 𝑡𝑎𝑟𝑔𝑒𝑡 𝑤𝑖𝑑𝑡ℎ 𝐿 𝑇 the path width and target width part of the equation. Repeat to students that H is a yes or no question. Either it hit, or it didn't. Don't count multiple hits on a single trial. Also, try to roll the marble as randomly as possible as to not bias the experiment.) Collect data and generate evidence to answer scientific questions: Carry out the investigation following the directions, gather and collect information. Reasoning Using mathematical expressions to represent phenomena to support explanations: Calculate the radius of the small marble, as well errors. (Teacher Tip: Measure and record the radius of the large marble so that you can share that with groups. A caliper is useful for this.) Communicating Investigate phenomena and describe the structure relationships: in small groups, answer the following questions: o Why are a large number of trials necessary for a measurement like this? o One student aimed while performing this experiment. Would his number of hits likely be larger or o o o smaller than expected? How would this effect his calculated marble radius? In Rutherford’s Experiment, only 0.005% of alpha particles bounced back. What does that indicate about the radius of the nuclei involved with the experiment? How does your measurement compare with the true measurement of the marble supplied by your teacher? List possible sources of error and how they might affect the calculated radius? List major problems that Rutherford and his colleagues faced when attempting to calculate the size of gold atoms. Assessment of Student Learning Completion of the assignment will be assessed, as will the accuracy of the calculations. Content will also be assessed in quizzes and the unit exam. SEP, CCC, DCI Featured in Lesson Science Essentials (Student Performance Expectations From Appendix C, D, E) Science Practices Developing and Using Models Using Mathematics and Computational Thinking Planning and Carrying Out Investigations Use representations to reflect on mechanisms of how things work. Using mathematical expressions to represent phenomena to support explanations. Collect data and generate evidence to answer scientific questions Crosscutting Concepts Scale, Proportion, and Quantity Structure and Function Use models to examine systems that are too small to observe directly Investigate phenomena and describe the structure relationships Disciplinary Core Ideas Structure of Matter HS-PS1-2 Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.