5. Artificial antigen
advertisement
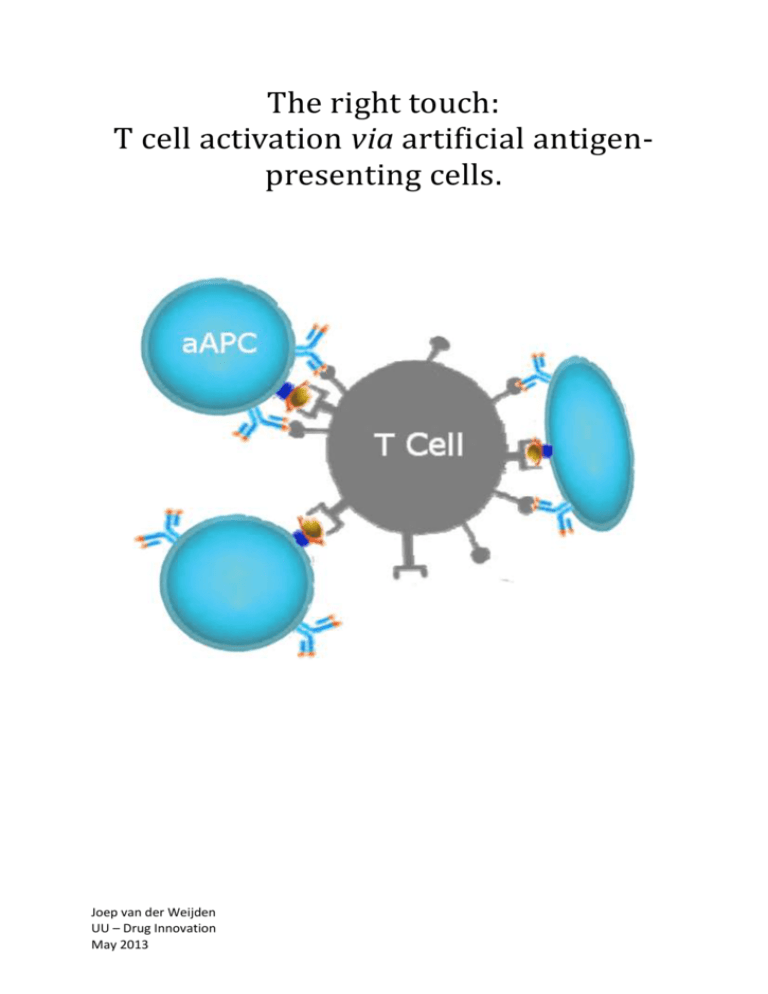
The right touch: T cell activation via artificial antigenpresenting cells. Joep van der Weijden UU – Drug Innovation May 2013 I. Abbreviations aAPC ACT APC CAR CCL CD CTL DC DN DP EPR effect GMP HLA ICAM-1 IFN-γ Ig IL IS Kd LFA-1 MHC (I/II) nLGs Ag PAMPs PBMC PEG PGA PLA PLGA pMHC PS RBC SLB SMAC TCR TGF-β Th TIL artificial antigen-presenting cell adoptive cell transfer antigen-presenting cell chimeric antigen receptor (C-C) motif ligand cluster of differentiation cytotoxic T cell dendritic cell double negative double positive enhanced permeability and retention effect good manufacturing practice human leukocyte antigen intercellular adhesion molecule 1 interferon gamma immunoglobulin interleukin immune synapse dissociation constant lymphocyte function-associated antigen 1 major histocompatibility complex class I/II nanosized liposomal polymeric gels antigen pathogen associated molecular patterns peripheral blood mononuclear cells polyethylene glycol polyglutamic acid polylactic acid poly(lactic-co-glycolic acid) peptide-major histocompatibility complex polystyrene red blood cells supported lipid bilayer supramolecular activation cluster T cell receptor transforming growth factor beta helper T cell tumor infiltrating lymphocyte 2 Table Of Contents I. Abbreviations ....................................................................................................................................... 2 1. Introduction ......................................................................................................................................... 5 2. The immune system[7,8] ....................................................................................................................... 6 2.1 Innate immunity ............................................................................................................................ 6 2.2 Adaptive immunity ........................................................................................................................ 7 2.3 Principles of T cell activation ......................................................................................................... 9 2.4 The immunological synapse ........................................................................................................ 11 3. Cancer immunotherapy..................................................................................................................... 15 3.1 Principles of cancer immunotherapy .......................................................................................... 15 3.2 Cancer immunotherapy via dendritic cells .................................................................................. 16 3.3 Cancer immunotherapy via adoptive T cell transfer ................................................................... 17 4. Biomimetic immunomodulation ....................................................................................................... 19 4.1 Biomimetic material design ......................................................................................................... 19 4.2 Physical and mechanical material properties.............................................................................. 20 4.2.1 Size........................................................................................................................................ 20 4.2.2 Shape .................................................................................................................................... 24 4.2.3 Rigidity .................................................................................................................................. 25 4.2.4 Zeta potential ....................................................................................................................... 25 4.3 Ligand presentation..................................................................................................................... 26 4.3.1 Presentation of surface bound ligands................................................................................. 26 4.3.2 Presentation of soluble ligands ............................................................................................ 27 4.3.3 Surface bound ligands that prolong circulation time ........................................................... 29 5. Artificial antigen-presenting cells ...................................................................................................... 30 5.1 Basic components of an aAPC ..................................................................................................... 30 5.2 Scaffolds used for artificial antigen presentation ....................................................................... 31 5.2.1 Liposomes ............................................................................................................................. 32 5.2.2 Supported lipid bilayers........................................................................................................ 32 5.2.3 Polystyrene beads ................................................................................................................ 33 5.2.4 Biodegradable particles ........................................................................................................ 34 5.2.5 Other scaffolds ..................................................................................................................... 34 6. Conclusions ........................................................................................................................................ 36 II. Summary............................................................................................................................................ 38 3 III. Samenvatting.................................................................................................................................... 39 IV. Bibliography ..................................................................................................................................... 41 4 1. Introduction The immune system constantly protects our body from a myriad of attacks from potentially lethal pathogens. Key to the success of our immune system lies in its ability to develop immunity to pathogens it has never encountered before. Professional antigen-presenting cells (APCs) known as dendritic cells (DCs) are vital in directing this so called adaptive immune response. Next to their role of protecting us from external pathogens, DCs are required for the generation of anti-tumor immunity[1,2]. If we could mimic DCs synthetically, we could potentially harness the power of the immune system for cancer immunotherapy. When a virus or bacteria manages to break through the skin, it is likely to encounter a dendritic cell. Via a process known as phagocytosis, the dendritic cell engulfs the pathogen and breaks down its structure. Next, it will present parts of the pathogens as antigens via a membrane protein known as the Major Histocompatibility Complex (MHC) on its cell surface. After the DC migrate to the lymphoid organs, this MHC-antigen complex is then used to activate T cells, a second set of specialized cells of the immune system capable of rapid and efficient killing of the pathogen or pathogen-infected cells. Similarly, the immune system scans the body for abnormalities in cellular secretion products, which is a potential sign of cancerous growth. When abnormal growth is detected, the cells responsible are removed. The generation of such anti-tumor immunity is mediated by DCs[1,2] and their function as APC has already successfully been exploited in the field of cancer immunotherapy. Unfortunately, the high costs of culturing each patients individual cells and processing them into a vaccine under good manufacturing practice (GMP) is a potential roadblock for clinical use[3,4]. The search for modular, off-the-shelf DC mimics has led researchers to the production of artificial antigen-presenting cells (aAPCs). A wide range of acellular scaffolds have been functionalized with the basic requirements for T cell activation[5] and consequently used to activate T cells. Choosing the right combination of scaffold and set of T cell activating ligands is vital to eliciting the right T cell response. Control over T cell activation makes aAPCs not only an attractive alternative to DCs in cancer immunotherapy, but also an important tool to investigate the factors that influence native T cell activation. The importance of scaffold morphology in intercellular signal transduction is increasingly recognized. Factors such as avidity or domain formation of presented signals on the one hand, and overall scaffold shape, rigidity and size on the other can influence the outcome of cellular signaling events. With this in mind, there have been increased attempts to use techniques from (bio)chemical engineering to more closely mimic natural signal transduction[6]. This review explores the role scaffold morphology in cellular interaction and signaling. Its focus will be on the biomimicry of APCs in the context of cancer immunotherapy using artificial scaffolds. To this end, the basic biology behind DC-based cancer immunotherapy is explained, followed by an overview of aAPCs described in literature. 5 2. The immune system[7,8] The immune system is the name given to the cells and molecules that protect our body from damage caused by infectious agents or other unwanted intrusion. An immune response is generated when the immune system recognizes an antigen as “non-self”. This sets the various effector cells and molecules of the immune system into action to clear the body of the antigen source. A wide range of pathogens are always recognized by the immune system, because they possess common antigens. These antigens trigger an innate immune response. Other pathogens, such as influenza, are more elusive and may not be recognized immediately. They require a more specific response (the production of antibodies, for example) in order to be eliminated. Such a response is called an adaptive immune response, because it requires the body to adapt to infection from that particular pathogen. An adaptive immune response is a form of immunological memory, a phenomenon gratefully exploited in vaccination. 2.1 Innate immunity White blood cells, or leukocytes, form the cellular part of both the innate and adaptive immune system, shown in figure 2. Leukocytes originate in the bone marrow and following maturation, they differentiate into more specialized cell types as they move out to the bloodstream, the lymphatic system and peripheral tissues. Essential cell types of the innate immune system are macrophages, granulocytes, mast cells and DCs. All of these are formed from the common myeloid precursor, a lineage of leukocytes. A common feature of these cells lies in their ability to engulf, or phagocytose, and destroy particles such as bacteria or even complete cells. The major population of leukocytes is called granulocytes, owing to their grainy staining when studied under a microscope. The peculiar staining comes from granules in their cytoplasm, which consist of a mixture of pore forming proteins capable of destroying microbes via lysis of the membrane. Granulocytes can be divided into three subtypes; eusinophils, basophils and neutrophils, of which the latter is the most abundant leukocyte in the blood. Macrophages, a mature form of monocytes shown in figure 1, are long lasting, phagocytic cells present throughout the body. Their primary function is to engulf and enzymatically degrade pathogens they may encounter and since they are distributed in most tissues, they are often the first line of defense of the innate immune system. In addition to the phagocytosis of microbes, macrophages act as the bodies’ janitor, clearing cells and cellular debris. By secreting signaling proteins, they can recruit other immune cells and induce inflammation, which is vital to a successful immune Figure 1. Left, a schematic representation of a response. macrophage. Right, a micrograph of a macrophage surrounded by leukocytes[169]. Dendritic cells (DCs) get their name from the finger-like protrusions, or dendrites formed by the cell membrane. Similar to macrophages and granulocytes, DCs engulf particles by phagocytosis. In addition, DCs 6 Figure 2. Dendritic cells form a key link between the innate immune system and the adaptive immune system. They are phagocytic and are specialized to ingest a wide range of pathogens and to display their antigens at the dendritic cell surface in a form that can be recognized by T cells[169]. continuously “drink” extracellular fluid in a process called macropinocytosis. In contrast to macrophages and granulocytes, DCs are specialized to process engulfed material intracellularly and present antigenic fragments through specialized receptors on their surface. This form of antigen presentation activates a type of cell from the adaptive immune system known T lymphocytes and gives DCs their role as a ‘bridge’ between the innate and the adaptive immune system. Although some other cell types such as macrophages are also capable of antigen presentation, DCs are the most potent type of APC[9]. The exact mechanism by which DCs present antigens to activate T lymphocytes is discussed in detail in section 2.3. 2.2 Adaptive immunity An important lineage from which many different cell types associated with the adaptive immune system arise is the common lymphoid progenitor. Collectively known as lymphocytes, these cells are mainly found in the blood and the lymphatic system. Most lymphocytes are antigen-specific cells, meaning that they can respond to one antigen only. The only exception comes from the natural killer cell (NK cell), which is specialized in killing tumor or virus infected cells. The remainder of the lymphocyte subtypes are antigen-specific. Because of the vast number of pathogens that our body might encounter during our lifetime, a huge repertoire of different antigenspecific cells exists in our body. This is possible through the modular way in which an antigen receptor is produced. By continuous recombination of the genes that code for the antigen receptor, our body can produce up to a billion different lymphocytes. Only when a lymphocyte is presented with the antigen specific to its antigen receptor, it goes from a naïve to an activated state. In the activated state, lymphocytes are called effector cells and perform various immunological functions in the body. There are two types of lymphocytes, the B cells and the T cells. B cells are most popularly known as the source for antibodies. After their antigen receptor recognizes an antigen, B cells transform into plasma cells or effector B cells. These cells produce and secrete large amounts of immunoglobulin (Ig) proteins, which is a soluble form of the antigen receptor on their cell membrane. This protein, 7 also known as an antibody, helps the immune system to recognize a specific pathogen, thereby promoting its removal from the body. Although T cells also recognize antigens through an antigen receptor (the T cell receptor, or TCR), their effector functions are different from B cells. T cells can differentiate into several different effector subtypes, which can be categorized as cytotoxic (specialized in killing), helper (stimulates other T cells and B cells) or regulatory (controls/suppresses other T cells). T cells can be found in the blood and the lymph. They differentiate from the common lymphoid precursor in the bone marrow, a primary lymphoid organ. From there, they migrate to the thymus where they mature. They then go on to secondary lymphoid organs such as the lymph nodes and the spleen where fully matured naïve T cells wait to be stimulated by antigenpresenting cells. The distribution of the different lymphoid organs through the body is shown in figure 3. When lymphocytes enter the thymus, they lack a functional T cell receptor nor do they express cluster of differentiation 4 (CD4) or CD8, both TCR co-receptors. At this stage, they are called double negative (DN) cells. As the DN cells proliferate, they start to express low levels of CD4, CD8 and the TCR, which consists of an α and β chain as Figure 3. The distribution of lymphoid tissues in the body. T cells arise from stem cells in bone marrow and differentiate in the thymus (yellow). They migrate from shown in figure 4. the thymus and are carried in the bloodstream to the peripheral lymphoid organs (blue), which include lymph nodes and the spleen. The peripheral lymphoid organs are the sites of lymphocyte activation by antigen, and lymphocytes recirculate between the blood and these organs until they encounter their specific antigen[169]. The incredible diversity of TCRs comes from the somatic recombination of the genes that code for the α and β chains respectively. This process, known as V(D)J recombination, is the origin of TCR diversity. After translation of the genes that code for the TCR, the full α:β heterodimer is formed at the surface of the cells, which are now called double positive (DP) cells because they express both the CD4 and CD8 coreceptor. Cells that engage with TCRs, such as DCs, do this through antigens Figure 4. Schematic structure of a T cell bound to MHC molecules on their surface. There are two classes antigen receptor. The TCR is composed of two chains, an α chain (yellow) and a β chain (green), each of which has a variable and a constant part. The variable parts of the two chains create a variable region, which forms the antigen-binding site[169]. 8 of MHC molecules; those that bind endogenous antigens (MHC class I) and those that bind extracellular antigens (MHC class II). To ensure that the newly formed TCRs function properly, they need to be able to bind to antigen/MHC complexes but avoid strong binding to self-antigens since that may lead to autoimmunity. The desired binding properties of TCRs is determined by positive and negative selection in the thymus. Positive selection ensures that the newly formed TCR has at least a weak affinity for an MHC complex. Double positive T lymphocytes that fail to bind an MHC complex through their TCR during positive selection die within 3-4 days. DP cells that survive positive selection will eventually become single positive (CD4 or CD8) T lymphocytes. During negative selection, apoptosis is induced in cells that bind too strongly to self-antigen/MHC complexes. Less than 5% of all DP cells survive this process, but such strict selection ensures that all single positive T lymphocytes that leave the thymus can recognize MHC and are self-tolerant. These matured cells, now called single positive (CD4 or CD8) naïve T lymphocytes, move to the periphery and end up in secondary lymphoid organs where they are maintained in low numbers until they meet “their” antigen and rapidly expand in numbers. This process is briefly illustrated in figure 5. Figure 5. Clonal selection. A single lymphocyte precursor gives rise to a large number of lymphocytes, each bearing a distinct antigen receptor. Lymphocytes with receptors that bind ubiquitous self antigens are eliminated before they become fully mature, ensuring tolerance to such self antigens. This is known as negative selection. When a foreign antigen interacts with the receptor on a mature naive lymphocyte, that cell is activated and starts to clonally expand. Antigen specificity is thus maintained as the clonally expanded cells proliferate and differentiate into effector cells. Once antigen has been eliminated by these effector cells, the immune response ceases, although some lymphocytes are retained to mediate immunological memory[169]. 2.3 Principles of T cell activation Mature, naïve T cells that circulate the blood and the lymphatic system need to be activated by antigen-presenting cells before they are fully functional. Once activated, T cells become effector T cells and perform various immunological tasks. Most nucleated cells display MHC molecules on their cell surface, but only DCs are capable of activating T cells. DCs continuously sample their surroundings for pathogens and depending on the way a pathogen enters the dendritic cell, the antigen fragments derived from the pathogens are loaded onto different types of MHC molecules. Extracellular pathogens that enter the cell through either receptor-mediated phagocytosis or macropinocytosis are loaded onto MHC class II receptors 9 and pathogens that are located directly in the cytosol (such as most viruses) are loaded onto MHC class I molecules after proteolytic degradation. However, some exogenous proteins internalized by DCs end up on MHC class I complexes, a phenomenon referred to as cross presentation. The distinction between peptide-MHC I (pMHC I) and pMHC II complexes on the APC surface is important because it determines the type of T cell that gets activated. After DCs capture an antigen, they move from the peripheral tissues like the skin to draining lymph nodes via lymphatic vesicles and mature[10]. In the lymph nodes, antigen presentation to naïve T cells takes place[11]. Activation of naïve T cells by APCs requires three signal, as illustrated in figure 6. The first signal in T cell activation is the recognition of a pMHC complex by a TCR on the T cell surface. The bond between a single pMHC:TCR is rather weak, but it can be stabilized by either CD4 or CD8, both co receptors of the TCR on the T cell surface. The pMHC II:TCR complex is stabilized by CD4 and the pMHC I:TCR complex is stabilized by CD8. Since single positive, naïve T cells express only one of the two co receptors, this gives rise to two different populations of effector T cells (CD4+ or CD8+) after activation. A second signal is provided by co stimulatory molecules on the APC, such as CD80 (B7.1) or CD86 (B7.2). These surface molecules interact with CD28 on naïve T cells. This second signal is needed for further activation of the T cell. Stimulation solely through the TCR, without co stimulatory signals, can lead to a state of anergy in the T cell. In this state, T cells are selectively non-responsive when presented with an antigen later on[12]. Adhesion molecules, such as the integrin LFA-1 on the T cell which interacts with ICAM-1 on the APC surface can be seen as another co stimulatory signal, since they serve to prolong and stabilize APC:T cell contact. A third signal comes from soluble signaling molecules, collectively known as cytokines. This signal influences to which effector cell a naïve T cell will differentiate. Figure 6. Three kinds of signals are involved in activation of naive T cells by antigen-presenting cells. Binding of the pMHC complex by the TCR and, in this example, a CD4 co-receptor, transmits an activation signal. Effective activation of naive T cells requires a second signal, the co-stimulatory signal, to be delivered by the same antigen- presenting cell (APC). For CD4 T cells in particular, different pathways of differentiation produce subsets of effector T cells that carry out different effector responses, depending on the nature of a third signal (arrow 3) delivered by the antigen-presenting cell. Cytokines are commonly, but not exclusively, involved in directing this differentiation[169]. The nature of the T cell response is strongly related to the nature of the presentation of the different activation signals. Activation of CD8+ T cells occurs through TCR recognition of a pMHC I complex (signal 1) on the APC and co stimulation through CD28 on the T cell surface. The effector cells of the CD8+ subtype are called cytotoxic T cells (CTLs) and these cells are capable of killing cells in an antigen-selective fashion. For example, when a CTL encounters a virus-infected cell presenting a viral peptide derived antigen on its MHC I complex, it will kill that cell. Activation of CD4+ T cells can lead to a variety of effector cells, of which the subtype is determined by the different cytokines (signal 3). When a naïve CD4+ T cell receives a signal 1 (through 10 pMHC II) and signal 2 from an APC in combination with the cytokine IL-12, it is likely to differentiate towards the T helper 1 (TH1) subtype. As the name implies, this effector type is important in aiding the (cellular) immune response by secreting another cytokine called IL-2, a potent T cell growth factor. Cells of the TH1 subtype are also efficient stimulators of macrophage activity, thereby increasing macrophage microbial activity. When IL-4 is present as a third signal, CD4+ T cells are prone to differentiate towards the TH2 subtype. T helper 2 cells are involved in humoral immune response by the activation of B cells. Another group of CD4+ T cells is called regulatory T cells, or Treg and these cells are important in preventing over-reactive CTLs from causing excess damage to tissues. One of the ways that regulatory T cells are induced is by antigen-loaded DCs which have failed to mature[13,14]. 2.4 The immunological synapse Here, we zoom in on the T cell:DC interaction to discuss the spatial and temporal elements of T cell activation and the minimal requirements for signal transduction. The site of signaling from a DC to a naïve T cell is known as the immunological synapse (IS), or supramolecular activation cluster (SMAC). This is a highly organized structure on the cell surface several micrometers across, with different stimulatory ligands clustered in spatially organized domains[15]. Figure 7. Structure of the immunological synapse. (A) The three layers on the T cell side of the immunological synapse are pictured here. The receptor layer contains the T cell receptor (TCR)–CD3 complex, CD4 or CD8, CD28 and leukocyte function-associated antigen 1 (LFA1). The signaling layer includes downstream phosphorylation proteins and the cytoskeletal layer contains mainly filamentous actin (F-actin). (B) The figure represents the supramolecular activation clusters (SMACs) of an immunological synapse, as seen looking down on a T cell. The TCR-rich central SMAC (cSMAC) core is shown in red, the CD28-rich cSMAC periphery is shown in green and the LFA1-rich peripheral SMAC (pSMAC) is shown in blue. The entire contact area between the T cell and antigen-presenting cell is outlined, and the yellow area represents Factin, which forms dynamic protrusions that move around the periphery of the synapse (the distal SMAC (dSMAC)) in a 11 radial wave. Each SMAC contains hundreds to thousands of receptors, and the dSMAC is the site at which TCR microclusters are first detected[16]. The interaction of a naïve T cell with a dendritic cell can be divided into distinct phases, based on the strength and duration of contact[17]. In the lymph nodes, naïve T cells constantly scan their surroundings in search for activating signals on DCs. In this scanning phase which can last from 30 minutes up to 8 hours, DCs are contacted by up to 5000 T cells in 1 h[18]. Initiation of contact between a DC and a naïve T cell occurs through the recognition of activating pMHC complexes in a sea of selfpMHC on the DC surface by the TCR. As few as 8-10 activating pMHC complexes can be enough to trigger a response (formation of a synapse) in T cells[19,20]. Following initial contact, activating TCR microclusters, enriched in costimulatory molecules such as CD28, move inward in an F-actin dependent manner to form the central supramolecular activation cluster (cSMAC). The central cluster of TCR-pMHC is surrounded by a ring of adhesion molecules known as the peripheral SMAC or pSMAC, which consists of adhesive contacts between LFA-1 on the T cell surface and ICAM-1 on the DC[21]. Together, the cSMAC and the pSMAC form a typical bull’s eye pattern, as shown in figure 7b. The interaction between a DC and a naïve T cell can last up to 8 hours and this is seen as a requirement of proper T cell activation. Finally, T cells return to their state of rapid migration and the activated T cell proliferates and exits the lymphoid system in search for its antigen in the periphery. From solution based affinity measurements we know that TCR:pMHC affinity is rather low, which is surprising given the highly specific interactions that occur between T cells and APCs. There are various explanations for this discrepancy, most of which have to do with the fact that solution-based affinity is not comparable to the in vivo situation. It is important to acknowledge the physical restraints of both TCR and pMHC, as illustrated in figure 8. These membrane bound proteins have limited degrees of freedom, which means that upon binding, the loss of entropy is less than what is to be expected from 3D, solution-based measurements[22,23]. In addition, the formation of TCR microclusters makes for a synergistic enhancement of single TCR:pMHC affinity[12,24]. The tight junction between the T cell and the DC at the IS, caused by the LFA-1 enriched pSMAC network also serves to enhance T cell sensitivity to antigen. Figure 8. Receptor-ligand kinetics in solution versus membranes. In theory, dissociation constants (Kd) for bound ligands in solution are calculated based on dissociation occurring in 3D space, with six degrees of freedom. However, when two opposed cell membranes are interacting, both receptor and ligand are confined to 2-D translation and 1-D rotation, which may stabilize and prolong these receptorligand interactions. Also, rebinding by neighboring receptors (such as clustered TCR) can further trap ligands and generate longer binding than would be predicted in solution[22]. The geometry of the immunological synapse is intriguing, since it adds contextual information to the traditional ligandreceptor view of signal transduction[25]. Many researchers have sought to image the IS, either by using ligands embedded in a supported lipid bilayer (SLB) to mimic the DC or by in vivo imaging. An antibody against one of the TCR coreceptors, CD3, shown in figure 7a, can be used to mimic 12 the pMHC complex since it will engage with TCRs and activate naïve T cells regardless of their antigen specificity. Figure 9. Immunological synapse spatial mutations. (A) Physical barriers to protein transport on a fluid supported lipid bilayer. Thin chrome lines create barriers to the diffusion of bilayer-tethered proteins (such as pMHC) and cellular proteins (such as TCRs) interacting with them (left). The spatial organization of the immunological synapse (TCRs; green) and ICAM1; red) without (1) and with (2–4) barriers of different geometries (right). (B) Subcellular size protein patterns functionalized on a surface. A TCR-activating antibody (anti-CD3; green) and an adhesion molecule (ICAM1; purple) are patterned on a flat surface (left). Anti-CD3, shown in schematics and cell overlays (in which anti-CD3 is blue) can be seen in a wild-type central zone pattern or in two variant patterns: multifocal and a peripheral ring (right). (C) The subcellular pattern of a TCRactivating antibody (anti-CD3ε; green) and a co-stimulatory antibody (anti-CD28; blue) on an adhesion molecule (ICAM1; purple)-rich surface (left). Different patterns are tested for their effect on T cell activation: TCR and CD28 follow the pattern of anti-CD3 and anti-CD28 antibodies, respectively, which can be either co-localized or segregated[26]. 13 Vale et al. were able to show TCR clustering and IS formation in Jurkat cells using anti-CD3ε and ICAM-1 tethered to a planar lipid bilayer, similar to the system shown in figure 9a[27]. Wind et al. performed a well-based single-cell study in combination with a cellular artificial APC to activate Jurkat cells and clearly show initial TCR clustering prior to colocalization to a single cSMAC[28]. By introducing constraints to the lateral mobility of the lipid-tethered ligands, or by patterning the ligands to a surface, it is possible to study the effect of spatial (re)organization on the T cell response. Some of these surface manipulations are shown in figure 9. From these studies, it was shown that TCR microclusters that are physically hindered to move towards the cSMAC continue to signal intracellularly and that spatial orientation of ligands strongly influences T cell response[16,26,29,30]. These studies are of importance when trying to mimic antigen-presenting cells synthetically, since they tell us which factors (i.e. ligand mobility, ligand density) are most influential for T cell activation. Although the studies presented here show the importance of spatial orientation of T cell activating ligands, we must to keep in mind that these systems are often incomplete mimics of what is happening in the native cellular environment. When a T cell is presented with pre-patterned ligands on an artificial surface, it will try to accommodate to the presented pattern, thus influencing the outcome of the experiment. Even though this is not the case when ligands are presented on a SLB surface without constraints, such SLB experiments might not translate completely to native T cell activation. Other factors, such as the choice of ligands that are used in these studies or temporal context in which they are presented to a T cell, might also affect the size and shape of the IS and thus the signal received by the T cell[22]. Direct, in vivo imaging of the IS, although not yet technically feasible, will provide a better picture of the importance of spatial organization in the IS[31]. Such information could be very useful for the construction of biomimetic aAPCs. 14 3. Cancer immunotherapy As stated by Palucka and Banchereau in 2012, “cancer immunotherapy attempts to harness the power and specificity of the immune system to treat tumors”[32]. The idea of using the immune system to combat diseases is not new. In fact, a major goal of vaccination is to induce T cell mediated long-lived immunity. The discovery that our immune system can recognize and eliminate cancerous cells in a DC-mediated manner[1,2], has inspired researchers to study the therapeutic effects of DCs and T cells in cancer. One example of a recent breakthrough in cancer immunotherapy is the full recovery of a patient with leukemia after treatment with chimeric antigen receptor (CAR) modified T cells[33]. 3.1 Principles of cancer immunotherapy Cancer cells often have altered protein expression levels compared to normal cells, which can result in the display of antigens that are associated with tumors. In order to establish antitumor immunity, the immune system has to complete several steps, illustrated in figure 10. First, a tumor associated antigen must be taken up by dendritic cells in an inflammatory context to ensure full maturation of the DCs. Next, these antigen loaded DCs must mature and find their way to the lymph nodes where they can interact with naïve T cells. The exact composition of T cell subtypes required to establish antitumor immunity is not known, but it is certain that it must include CD8+ cytotoxic T cells (CTLs) [34]. In the presence of an inflammatory signal, such as IL-12, these CD8+ T cells develop into a memory subtype which persists long after [35] activation . Although most of the work on T cell mediated cancer immunotherapy has focused on the generation of CTLs, the CD4+ T cell subtype has an important role in sustaining an active immune response by secreting the [36] stimulatory cytokine IL-2 . After the establishment of an activated repertoire of tumor-antigen specific T cells, the CTLs have to reach the tumor site where they can execute their cytotoxic function. Several factors hamper the proper functioning of CTLs in the tumor microenvironment, such as limited Figure 10. Generation and regulation of antitumor immunity. Antitumor immune responses must begin with the capture of tumor-associated antigens by dendritic cells, either delivered exogenously or captured from dead or dying tumor cells. The dendritic cells process the captured antigen for presentation or cross-presentation on MHC class II and class I molecules, respectively, and migrate to draining lymph nodes. If capture and presentation occurred in the presence of an immunogenic maturation stimulus, dendritic cells will elicit anticancer effector T cell responses in the lymph node; if no such stimulus was received, dendritic cells will instead induce tolerance leading to T-cell deletion, anergy or the production of Treg cells. In the lymph node, antigen presentation to T cells will elicit a response depending on the type of dendritic cell maturation stimulus received and on the interaction of T-cell co-stimulatory molecules with their surface receptors on dendritic cells. Antigen-educated T cells will exit the lymph node and enter the tumor bed, where a host of immunosuppressive defense mechanisms can be produced by tumors that oppose effector T cell function[34]. 15 expression of tumor-antigen presenting MHC I molecules by the cancer cells and the presence of immunosuppressive cytokines. To help the immune system to generate an effective immune response against cancer, two fundamentally different promising approaches have evolved. One approach is aimed at the delivery of (exogenous) tumor antigen to dendritic cells to enhance antigen presentation to T cells, while the other focuses on ex vivo activation and expansion of patient-derived naïve T cells, followed by reinfusion of these cells. The latter process is also known as adoptive cells transfer, or ACT. Both approaches are discussed in more detail below, as they form the therapeutic context for the development of aAPCs. 3.2 Cancer immunotherapy via dendritic cells Dendritic cells are capable of capturing tumor antigens and cross present them to naïve T cells in the lymph nodes to induce an anti-tumor CTL response[1,2], a process which can be exploited in cancer immunotherapy as illustrated in figure 11. Within dendritic cell-based cancer immunotherapy there are two approaches to increase antigen presentation by DCs in vivo. One approach is to grow DCs from patient-derived monocytes ex vivo, load them with the antigen of interest and then inject them back into the patient. The tumor antigens can be introduced by culturing the DCs in the presence of the antigen; when added in excess, the tumor antigen will Figure 11. Antigen capture and presentation by antigen-presenting cells. simply exchange with the antigens Tumor-associated antigens are exogenously delivered to provide a source of already present on the MHC antigen. Antigens can then be taken up by antigen-presenting cells, expressing co stimulatory molecules such as CD80, to be processed and molecules on the DC surface. As an presented to T cells. Phenotypes of CD4+ T cells can either enhance (eg, Th1) alternative to coculturing the DCs or inhibit (eg, Treg) the immune response[46]. with a single antigen, tumor cell lysates can be added to the culture medium to allow DCs to process tumor antigens through natural pathways. Although this ex vivo approach has been widely explored[32,37], the labor required to generate antigen-loaded DCs for each individual patient and the limited lifetime of DCs in culture (a few days), limits its broad applicability to a clinically relevant setting[3]. Antigens can also be delivered directly to DCs in vivo using targeted delivery of antigen to peripheral DCs. It was shown by Steinman and colleagues that efficient delivery of antigen to DCs and consequent CTL response is possible by coupling the antigen to a DEC-205 antibody, a DC specific surface protein[38]. Other means of targeted antigen delivery include, but are not limited to; antigen- 16 loaded, biodegradable polymeric nanoparticles[39,40], antigen-coupled surface modified polymeric particles[41] or magnetic particles[42]. Often in DC targeting, a danger signal or adjuvant is provided along with the antigen in the form of an immunogenic carrier or directly through the coadministration of the cytokine IFN-γ. Collectively, such immunogenic danger signals are known as pattern-associated molecular patterns (PAMPs). Without such a danger signal, targeted DCs may become tolerogenic, inducing regulatory T cells that may hamper an effective immune response[14,43]. Overall, DC based cancer immunotherapy is far from being commonly used as a treatment modality for cancer. Due to the complex nature of T cell activation by DCs, control over DC maturation and consequent T cell activation at the level of DCs is poor. 3.3 Cancer immunotherapy via adoptive T cell transfer The adoptive transfer of ex vivo stimulated, tumor-antigen specific T cells, a therapeutic approach illustrated in figure 12, bypasses the difficult task of dendritic cell manipulation in vivo, where a suppressive tumor micro-environment can interfere with DC development. For example, some tumors are infiltrated by a population of cytotoxic T cells, but due to the immunosuppressive microenvironment generated by the tumor, these tumor infiltrating lymphocytes (TILs) are not capable of eliminating the cancerous cells. Systemic administration of IL-2 is known to stimulate T cell growth and survival, but the high dose required to expand TILs in vivo often leads to significant toxicities. Combining these findings, researchers have successfully expanded populations of TILs ex vivo from a tumor biopt by adding IL-2 to the culture medium[44]. The adoptive T cell transfer (ACT) of such ex vivo expanded TILs has shown to be efficient in the treatment of patients with melanoma[45]. Figure 12. Isolation and ex vivo stimulation of antigen-specific tumor-reactive T cells for adoptive cell transfer. Autologous lymphocytes are stimulated ex vivo with either antigen-loaded dendritic cells or artificial antigen-presenting cells. Enriched tumor-specific T cells are then re-infused for treatment[46]. 17 Genetic manipulation of ex vivo cultured T lymphocytes to provide them with antigen-specificity provides another interesting therapeutic opportunity for ACT[47]. The previously mentioned success with CAR-modified T cells[33] is an example of an ACT-based therapeutic intervention. For the genetic manipulation of T cells, peripheral blood mononuclear cells (PBMCs) are harvested from a patient and non-specifically expanded ex vivo by repeated stimulation with anti-CD3 and anti-CD28 antibodies. When the cells are grown to the desired number, they are transfected with the gene of interest using a viral delivery system after which they are reinfused. The potential beneficial outcome of ACT is greatly improved if the ex vivo cultured T cells are infused only after lymphodepletion, in combination with a dose of IL-2. Lymphodepletion reduces the presence of negative regulators for inflammation, prolonging survival and enhancing the effector functions of the adoptively transferred cells[48]. Antigen-presenting cells can be used to generate antigen-specific T cells from a polyclonal population of PBMCs (figure 12). Patient-derived, antigen-pulsed DCs have been used in this way to generate effector T cells for the treatment of melanoma[49,50]. However, DCs are not ideally suited for ex vivo culture due to their short life-time and batch-to-batch variations. Moreover, autologous DCs may be dysfunctional due to the diseased state of the donor. To overcome this problem, T cells have been clonally expanded using aAPCs[5,51], an approach which is also illustrated in figure 12. The use of aAPCs to clonally expand T cells ex vivo allows for a high degree of control over the signals 1,2 and 3 that are presented to the T cells, which makes this technique not only well suited for the production of antigen-specific T cells for ACT under GMP, but also for more fundamental research on T cell activation. Chapter 5 covers various artificial antigen-presenting systems in more detail. Adoptive cell transfer shows great promise in the prevention and treatment of cancer, with numerous clinical trials performed and many more on the way[52]. However, ACT is far from becoming a routine clinical practice as its implementation up to now is laborious and costly. In this regard, the use of ACT in cancer immunotherapy would greatly benefit from the development of a well defined, cost-effective “off-the-shelf” aAPC platform for the ex vivo expansion and activation of T cells. 18 4. Biomimetic immunomodulation Advances in material engineering provide an opportunity for tailor made biomimetic signaling platforms. In this chapter, we look at recent advances in material chemistry and how material properties such as size and shape affect interaction with biological systems, specifically in the context of T cell activation. In addition, we discuss the various approaches to ligand presentation on artificial scaffolds. 4.1 Biomimetic material design Biomaterials can provide a temporal and spatial context for the delivery of information, something which is especially important in intercellular communication. The biomimetic properties of materials have traditionally been limited by available chemistries, but advances in biomaterial synthesis now allow us to effectively incorporate lessons from nature into material design[53,54]. Differences in physical properties of natural objects, such as size and shape, are obvious when observed at the macroscopic scale, but such properties are less well appreciated at the microscopic scale. Figure 13 illustrates the extreme diversity in shape encountered at the microscopic level in nature, ranging from viruses to pollen and the immune synapse. In bacteria alone, numerous examples can be found in which shape and size contribute to biological function[55]. Figure 13. Examples of natural biological objects that have diverse physical properties. (A) Human herpesvirus; scale bar, 100 nm (image: Frank Fenner). (B) Ebola virus; scale bar: 500 nm (image: Frederick A. Murphy). (C) Enterobacteria phage λ; scale bar: 50 nm (image: University of Wisconsin-Madison). (D) Human erythrocytes; scale bar approximately 10 μm. (E) Escherichia coli; image size approximately 7 μm x 6 μm. (Panels D and E © Dennis Kunkel Microscopy). (F) Surface texture in alveolar macrophages; scale bar: 5 μm (© 2008 STM). (G) Pollen; image size approximately 50 μm x 45 μm (Dartmouth Electron Microscope Facility). (H) Intestinal villi; approximate magnification x5,950 (© Dennis Kunkel Microscopy). (I) The immunological synapse; T cell forming a synapse with a supported membrane containing GPI-linked pMHC and ICAM; © 1999 AAAS). (J) A schematic of cellular compartmentalization showing several organelles surrounding the nucleus. The images clearly establish that nature uses physical parameters such as size, shape, texture and compartmentalization in designing life[53]. Smart biomimetic material design has taken lessons from nature and applied it to for example, drug delivery, where biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) enable the slow release of bioactive molecules, and particle coating with polyethylene glycol (PEG) prolongs circulation time and minimizes non-specific interactions. Targeted drug-delivery with PEGylated nanoparticles is common practice in research and has led to clinically approved drug formulations already two decades ago[56]. In the context of immunotherapy, it is vital that the biomaterial, which 19 usually serves as a carrier, is immunologically non-reactive, meaning it is not recognized by the immune system. As we increase our understanding of biological systems on the one hand, while adding complexity to synthetic systems on the other, we are slowly able to bridge the gap between the synthetic and the biological world, as illustrated in figure 14). This hold great promise for immunology, where many translational challenges like synthetic implants, tissue engineering and tools for (cancer) immunotherapy can benefit from advances in (bio)engineering. Figure 14. Bioengineered, bio-inspired and biomimetic systems. The gap between synthetic and biological systems has traditionally been very large. However, recent advances in the synthesis of novel materials and understanding of biological systems have paved the way towards bridging this gap. Combining perspectives from the synthetic and biological fields will provide a new paradigm for the design of immunological tools. PEG, polyethylene glycol[54]. 4.2 Physical and mechanical material properties Smart material engineering allows us to explore open questions in basic immunological function, specifically how dendritic cells and T cells interact. Recently, the properties of materials used for immunomodulation has been extensively reviewed[6,57–59]. Here, we discuss the findings from these reviews and relate those findings to T cell stimulation and in addition, consider which signals are appropriate for T cell activation and what would be the effect of avidity and paracrine delivery of such signals. Ultimately, this information should provide a picture to which material properties are especially important in T cell activation by artificial antigen-presenting cells. 4.2.1 Size Size matters. At least when it comes to the way in which micro- and nanoparticles interact with their surroundings. The profound effect of particle size on its interaction with its surroundings has been observed at the cellular level in terms of endocytosis and signal transduction, but also in body biodistribution and in vivo motility. All cells are capable of engulfing extracellular material through processes collectively known as endocytosis, shown in figure 15. Endocytosis provides a means for cells to absorb nutrients and probe their surroundings. There are some size restrictions to the different endocytotic pathways; while most cells are only capable of ingesting sub-micron sized particles by pinocytosis, only phagocytotic cells readily take up particles that are over one micron in size[60]. However, endocytosis of particles larger than 1 µm has been shown for non-phagocytotic cells. Gratton et al. showed uptake of solid microparticles up to 3 µm in size after incubation with cervical cancer cells (HeLa)[61]. Particle size is well known to influence cellular uptake, which is illustrated by the rate of phagocytosis 20 of polystyrene microparticles by macrophages[62]. This was shown to be size-dependant and maximum phagocytosis was observed at a particle diameter of 2 to 3 µm, which interestingly coincides with the typical diameter of bacteria. For nanoparticles in the range of 30-100 nm, with ζpotentials ranging from -23 mV to +9 mV, size is the major determinant for non-specific uptake by macrophages, where 100 nm particles are taken up fastest[63]. The rate of particle uptake is of importance in intercellular signaling, since slower uptake usually more time for the particle to reach its target. Figure 15. Modes of cellular internalization of nanoparticles and respective size limitations. (A) Internalization of large particles is facilitated by phagocytosis. (B) Nonspecific internalization of smaller particles (>1 μm) can occur through macropinocytosis. (C) Smaller nanoparticles can be internalized through several pathways, including caveolarmediated endocytosis, (D) clathrin-mediated endocytosis and (E) clathrin-independent and caveolin-independent endocytosis, with each being subject to slightly different size constraints. Nanoparticles are represented by blue circles (> 1 μm), blue stars (about 120 nm), red stars (about 90 nm) and yellow rods (about 60 nm) [56]. At the cellular level, particle size may also influence signal transduction when the particle is coated with signaling molecules, something that is evident from T cell stimulation with artificial antigenpresenting cells. Microparticles are better at activating T cells than nanoparticles coated with the same stimulatory ligands[64,65]. This may be explained by the requirement of a large, continuous surface for the formation of a mature immune synapse, a structure several microns in diameter. 21 The in vivo biodistribution and clearance of particles is largely dictated by the particle size and the route of administration[66]. In general, small particles (<100 nm) show increased in vivo mobility compared to larger particles, which is due to the ability of smaller particles to pass through natural barriers in the body. On the other hand, particles over 5 µm in diameter are likely to cause embolism after intravenous administration[67], since their circulation is limited by the diameter of the smallest capillaries (2-3 µm). The passive targeting of nanoparticles is often exploited in cancer therapy, where it is known that particles up to 100 nm often preferentially leave the vasculature at sites of inflammation, due to the enhanced permeability and retention (EPR) effect. In healthy tissues, particles between 100-200 nm are optimally suited for prolonged circulation, since they are large enough to avoid uptake in the liver, but small enough to avoid filtration in the spleen[56]. However, non-rigid, red blood cell like particles show prolonged circulation even at sizes up to 5 µm, probably due to their ability to deform when passing through biological barriers[68]. The lymphatic system is an important size-dependant targeted organ for immunomodulation, the full dynamics of which is illustrated in figure 16. After intradermal administration, nanoparticles are known to reach the draining lymph nodes in a size dependant manner through the interstitial fluid, with an upper size limit of around 100 nm dependant on tissue and injection pressure. In DC targeted immunotherapy, passive migration of sub-100 nm nanoparticles to draining lymph nodes is often used to deliver antigen to dendritic cells[39,69–71]. Although particles up to 180 nm can also reach the draining lymph nodes after subcutaneous administration, this can take up to several days[72]. Recently, Hubbell and coworkers showed that OVA-antigen coupled to a solid core, 30 nm nanoparticle via a reduction-sensitive linker elicited a CD8+ T cell response whereas OVA-antigen encapsulated in 125 nm polymeric vesicles (polymersomes) preferentially elicited a CD4+ T cell response after subcutaneous administration[71]. 22 Figure 16. Tissue and cell targeting for antigen delivery in nanoparticulate subunit vaccines. (A) Nanomaterials injected into most tissues flow into draining lymphatic capillaries or are taken up by tissue-resident APCs. Small particles (red, <100 nm) more easily penetrate the extracellular matrix and thus distribute more broadly than larger particles (orange), which are retained more in the tissue. Interstitial APCs can be exclusively targeted by particles that are >500 nm or decorated with targeting antibodies (blue). Upon entering lymphatic vessels, cells and antigens travel to the draining lymph node. (B) Inhaled or consumed antigens or nanomaterials mostly target the nasal- or gastrointestinal- associated lymphoid tissue (NALT or GALT, respectively), which include Peyer’s patches. Because of the tight epithelial barrier, most antigens and particulates are taken up by immune cells, where they then traffic to the lymph node. (C) Antigens, particulates, and immune cells from the afferent lymphatics encircle the lymphnode in the subcapsular sinus and enter different areas according mainly to size. Subcapsular macrophages take up large particles (>7 nm) or opsonized antigens (orange), whereas smaller antigens flow into conduits (red). (D) Polymeric nanoparticles (<50 nm, red) are rapidly cleared after intradermal injection and drain into the lymph node, where they are taken up by APCs surrounding the lymphatic endothelium (blue) and T cell stroma (green). Scale bar: 200 mm. (E) Multilamellar lipid vesicles (180nm,blue) were drained into the lymph node, after which germinal centers (green) formed nearby, generating B cells (red) specific to the antigen borne by the vesicles. Scale bar 200mm. (F) APCs collect polymer nanoparticles (<50nm, red) in the airways (lung parenchyma, blue) and migrate to the pulmonary lymph node. Scale bar: 10 mm[58]. 23 4.2.2 Shape Although it has long been known that viral and bacterial shape influences in vivo behavior, the systematic study of shape-effects on the behavior of micro- and nanoparticles has only recently gained attention with the advent of techniques that allow for the synthesis of non-spherical particles[73,74]. The shape of micro- and nanoparticles can have a profound effect on uptake by phagocytotic[75–78] and non-phagocytotic cells[61,79]. As a rule of thumb, particles with a higher aspect ratio show slower uptake, independent on particle size[78] or cell type[79]. As a high aspect ratio particle is more likely to approach a cell with one of its low curvature sides, extensive actin remodeling, an energy dependent process, is required to internalize the particle. This can help to explain the observations made by Sharma et al., who found that ellipsoid polystyrene microparticles attached more readily to macrophage surfaces than their spherical counterparts, but are taken up less efficiently[75]. The sizeindependent, aspect ratio-dependent uptake of micro- and nanoparticles by macrophages as found by Champion et al. is illustrated in figure 17. Particle shape also significantly influences the circulation time in vivo, an effect which can be observed from the disk shaped red blood cell (RBC), which can stay in circulation up to 120 days. Although the prolonged circulation time observed with RBCs is not only due to its shape, prolonged circulation of synthetic microparticles that mimic the dimensions close to those of RBCs has been observed[80]. Geng et al. found that elongated micelles (filomicelles) show circulation times of up to a week, which was significantly longer than their spherical counterparts[81]. A tempting explanation for the prolonged circulation time of disks and filomicelles is their ability to align with the blood stream, which would minimize contact with the cell wall and phagocytotic cells present in the blood[81]. Figure 17. Scanning electron micrographs (A-C) of particles during phagocytosis and (D) a phagocytosis phase diagram. Top: Micrographs (A–C) of cells and particles were colored brown and purple, respectively. (A) The cell body can be seen at the end of an opsonized elliptical disk, and the membrane has progressed down the length of the particle (Scale bar: 10 µm). (B) A cell has attached to the flat side of an opsonized elliptical disk and has spread on the particle. (Scale bar: 5 µm). (C) An opsonized spherical particle has attached to the top of a cell, and the membrane has progressed over approximately half the particle. (Scale bar: 5 µm). (D) Phagocytosis phase diagram with Ω and dimensionless particle volume V* (particle volume divided by 7.5 µm radius spherical cell volume) as governing parameters (n = 5 for each point). There are three regions. Cells attaching to particles at areas of high Ω, >45°, spread but do not initiate internalization (region C). Cells attaching to particles at areas of low Ω, <45°, initiate internalization (regions A and B). If V* is ≤1, internalization is completed (region A). If V* > 1, internalization is not completed because of the size of the particle (region B).The arrows above the plot indicate the point of attachment for each shape that corresponds to the value of Ω on the x axis. Each case was classified as phagocytosis or no phagocytosis if >95% of observations were consistent. Each data point represents a different shape, size, or aspect ratio particle. Adapted from[78]. 24 Particle shape may also influence signal transduction, since some signaling events, like DC:T cell interactions, exhibit clear shape dependency. In a comparative study, spherical, rod- and disk-shaped polystyrene micro- and nanoparticles were coated with the breast cancer cell recognizing antibody trastuzumab and assessed for specific and nonspecific uptake. Rods exhibited higher specific uptake and lower nonspecific uptake in all cells compared with spheres, implying that there is an increased binding to rod-shaped particles compared to spherical particles[82]. This could be of interest to microor nanoparticles mediated cellular signaling or delivery of cytotoxic drugs to cancerous cells. 4.2.3 Rigidity One mechanical property that is often overlooked in biomimetic material design is the rigidity of the material, although this property may affect cellular interaction in a number of ways. In an attempt to mimic red blood cells, Doshi et al deposited layers of protein on a preformed, RBC shaped PLGA template. After cross linking of the protein layer, the PLGA template was dissolved, leaving a hollow, flexible synthetic RBC (sRBC) with an elastic modulus close to that of mouse RBCs. By virtue of their flexibility, these sRBCs are able to pass through microchannels with a diameter below that of their resting diameter[83]. In a study with soft and rigid polyacrylamide microparticles, macrophages showed a strong preference to engulf the rigid particles[84]. Using the Particle Replication in Nonwetting Templates (PRiNT) technology, Merkel et al. were able to produce sRBC with a finely tuned elastic modulus ranging from 8-64 kPa, depending on the degree of cross-linking. After in vivo administration, the particles showed differences in circulation time dependent on their elastic modulus, with an eight-fold decrease in circulation time correlating to a 30-fold increase in circulation time[68]. With regard to ligand recognition, substrate adhesion by ligand-coated polyethylene oxide-bpolybutadiene polymersomes was found to be lower for flaccid polymersomes compared to their more rigid counterparts[85], and in the context of T cell activation, stiff polyacrylamide gels incorporating anti-CD3 and anti-CD28 were more effective at T cell activation than soft gels, measured by IL-2 secretion[86]. 4.2.4 Zeta potential Most mammalian cells exhibit a negative overall charge on their surface due to the presence of sialic acids in the carbohydrate coating of the cellular membrane. As a consequence, the ζ-potential, a measure for the potential difference between a (particle) surface and its surroundings, can be used to predict the behavior of a particle when it encounters a cell. Positively charged particles show stronger non-specific interaction with cells[61,63,87] and can penetrate the cell membrane, a property exploited in drug delivery. These findings have an impact on biomimetic particle design; for prolonged circulation and minimal non-specific interaction, a particle must preferably have a ζ-potential that is neutral or slightly negative. As reactive functional groups such as thiols, amines and carboxylic acids strongly influence surface charge, care must be taken to control the display of these functional groups on the particle surface. Specifically for particles functionalization, it could be useful to select non-charged functional groups ligand attachment[87]. Soluble proteins found in serum will affect the net charge of by forming a protein corona around the particle, so for any biological application the ζ-potential must be determined only after incubation with serum[63]. 25 4.3 Ligand presentation In the classical approach to ligand-receptor interactions, both the ligand and receptor are considered to be free in solution, whereas in cell to cell communication usually at least either the ligand or the receptor is bound to a cellular membrane. Intercellular signaling events involve multiple ligandreceptor interactions, examples of which include immunological and neuronal synapses[88]. Here, we will discuss both surface bound and soluble ligand presentation, specifically for T cell activation and prolonged circulation. 4.3.1 Presentation of surface bound ligands In intercellular communication processes, such as immune recognition or cellular adhesion, multiple surface bound ligands are often presented to enhance signal strength. The combined strength of multiple binding interactions is known as avidity, not to be mistaken with the affinity associated with a single ligand-receptor interaction. An excellent example of the power of multivalent binding is found in the significant (100-fold) increase in affinity of TCR:pMHC binding in situ vs TCR:pMHC binding in solution[89]. In fact, the interaction between a single TCR and peptide-MHC complex is very weak, with a dissociation constant (Kd) in the order of 1-100 µM resulting in the requirement of multiple pMHC complexes to stimulate T cells[24]. The power of multivalent binding has led to the development of scaffolds that can display multiple ligands[90]. Such scaffolds can be anything from proteins to dendrimeric polymers or solid particles. Figure 18. Three ligands (recognition (red), costimulatory (green), and adhesion (blue)) that could be presented on the surface of artificial antigen presenting cells (aAPCs) in various ways. (A) Isotropic surface presentation of randomly distributed ligands. All three ligands are presented uniformly over the particle surface. (B) Anisotropic presentation of ligands in a patch pattern on the surface of a particle. Recognition and costimulatory ligands are randomly distributed in the patch, with a surrounding field of adhesion ligands. (C) Dynamic anisotropic presentation of ligands on a fluid supported lipid bilayer (SLB; yellow). Before interactions with a cell (e.g., T cell), lower initial surface densities of randomly distributed ligands may be placed on the SLB surface. Anisotropic reorganization of ligands occurs in response to interactions with a cell. The resulting bull’s eye pattern would be characteristic of a natural supramolecular activation clusters (SMAC) formed at immune synapse between a T cell and APC[6]. Ligands can be bound to a scaffold in a random fixed (isotropic),site selective fixed (anisotropic) or dynamic fashion, illustrated in figure 18. The ability of ligands to move in response to a binding event can be crucial for signal transduction, and this dynamic remodeling of surface bound ligands is increasingly recognized as an important aspect of cellular communication[91]. Although working with solid micro- and nanoparticles is more appealing than liposomes from an engineering point of view due to the poor stability of liposomes[92], solid particles only allow for the attachment of fixed surface bound ligands. An elegant solution for this problem is to coat solid particles with a lipid 26 bilayer, effectively mimicking the fluid nature of natural cell membranes. Ashley et al. coated silica nanoparticles with a lipid membrane that was targeted to hepatocellular carcinoma[93]. A combination of targeting and fusogenic peptides bound to lipids in the bilayer ensured rapid uptake and release of the drug-loaded silica core inside the targeted cells. The influence of the fluidity of the spherical supported lipid bilayer (SLB) on binding to Hep3B cells was investigated using either a Figure 19. Recruitment of Alexa Fluor 647-labelled fluid DOPC or an non-fluid DPPC lipid for the construction SP94 peptides (white) to the surface of a Hep3B cell of the SLB. At similar targeting peptide concentrations when peptides are displayed on a (~6 per particle), a 100-fold increase in binding affinity nitrobenzoxadiazole-labelled SLB (green) composed of DOPC (open circles) or DPPC (closed circles). was observed when the fluid DOPC was used to construct These data were collected at 4°C. Hep3B cells were the SLB. This dramatic increase in binding was attributed labeled with CellTracker Red CMTPX (red) and Hoechst 33342 (blue). Inset scale bars=5 µm[93]. to the ability of ligands in the fluid SLB to reorganize, as shown in figure 19. Similarly, lipid coated silica microparticles that were designed to capture viral particles through multiple ligand-receptor interactions showed a reduction in captured viral particles at a lower temperature due to reduced lateral mobility of the ligands in the SLB[94]. The ability of ligands to dynamically reorganize in response to a binding event has been studied for leukocyte mimics, or leuko-polymersomes (bilayer vesicles constructed of amphiphilic block copolymers)[95]. Surface bound ligands can be attached to particles via different synthetic strategies and an optimal ligation strategy most be chosen for each type of ligand-particle pair. A discussion of these strategies is outside the scope of this thesis, but the reader is referred to a recent review on the topic[96]. 4.3.2 Presentation of soluble ligands Cells can communicate with their environment via paracrine signaling, a process that describes the release of soluble factors, such as cytokines and chemokines, by cells into their surrounding in order to elicit a response from neighboring cells. Cells use paracrine signaling in processes such as tissue regeneration, neuronal signaling and immunity. Paracrine signaling allows for a local, high concentration of signal, without the need for direct contact, as illustrated in figure 20[97]. Moreover, paracrine release allows cells to create a signal gradient, which other cells can home to or move away from. The biomimetic release of soluble protein-based ligands in a paracrine fashion was first shown in 1976[98] and has evolved significantly with the design of novel biodegradable polymers such as poly(lactic-co-glycolic acid (PGLA)[99,100] and other systems, recently reviewed by Rothstein and Little[101]. Here, we discuss how the presentation of soluble ligands and in particular paracrine signaling can steer biomimetic immunomodulation. Figure 20. Cytokine release from a paracrine signaling antigen-presenting cell (paAPC). IL-2 release from paAPCs in isolation and in the vicinity of T cells was modeled using a diffusion equation to illustrate the level of IL-2 accumulation that T cells experience during initial interaction with paAPC. Left , a T cell paAPC interaction at 3 µm apart and right, 20 nm apart[97]. 27 Collectively, signaling molecules released by cells are called cytokines. Some cytokines, specifically those released by APCs, strongly influence T cell maturation and differentiation. During T cell development, dendritic cells secrete cytokines to promote T cell growth and differentiation towards a specific lineage[102]. It should come as no surprise then, that these cytokines have been tested for therapeutic applications, either by systemic administration or as adjuvant in vaccines[103]. The toxicity of high-dose IL-2 therapy, which was previously discussed, has promoted research into the biomimetic release of IL-2 and other soluble factors for therapeutic applications to enable high local concentrations of the cytokine while avoiding toxic side effects[104–109]. In one recent example, Fahmy and coworkers described the combined paracrine release of a TGF-β inhibitor and IL-2 from nanosized liposomal polymeric gels (nLGs)[108]. The TGF-β inhibitor serves to counteract the immunosuppressive function of TGF-β in the tumor microenvironment, allowing IL-2 to optimally stimulate cytotoxic natural killer and CD8+ T cells. Evaluation of these nLGs in a melanoma mouse model revealed a delayed tumor growth, increased survival of tumor-bearing mice, and increased the activity of natural killer cells and of intratumoral-activated CD8+ T cell infiltration[108]. Paracrine release of IL-2 has also been explored for the non-specific stimulation of naïve T cells. Naïve T cells were cultured in the presence of a non-specific activation signal provided by aCD3/aCD28 coated beads and either soluble or paracrine delivered IL-2. The slow, sustained release of IL-2 from microparticles significantly increased CD8+ T cell proliferation in comparison with exogenously added IL-2[104]. This demonstrates that a local cytokine microenvironment created by paracrine release can provide additional information for T cell development. In addition to cytokines, some cells also secrete chemokines to recruit nearby cells to for instance a site of inflammation. Local release of chemokines can be used to manipulate cell trafficking in vivo and has been explored for therapeutic applications, including recruitment of TILs[110], DCs[111] and regulatory T cells[112]. Recruitment of cells could be interesting for vaccination strategies, in which one could image a local depot of antigen being injected together with a chemokines as attractant for Figure 21. Migration paths of T cells chemotaxing toward CCL21 chemokine-releasing microspheres (CRMs). Resting or activated human T cells were embedded in collagen gels and imaged by videomicroscopy for 1.5 h. In parallel, control samples of T cells and empty CRMs in collagen mixed with 10 μg/mL “free” CCL21 were imaged. Shown are single-cell paths for cells whose starting positions (ro) were greater than or less than 100 μm from the nearest bead, color-coded by cell motility[114]. 28 dendritic cells and naïve T cells to form a synthetic lymph node[113]. Leukocyte migration in alginate gels was studied using microbeads that secreted CCL19 or CCL21, showing either leukocyte colocalization with or “hopping” from bead to bead, dependant on the chemokine concentration and release rate[114]. Figure 21 shows the migration of activated and resting T cells towards CCL21 releasing microspheres. 4.3.3 Surface bound ligands that prolong circulation time After administration, micro- and nanoparticles are often cleared from the bloodstream by phagocytes before they reach their desired target in the body. This has motivated researchers to find ways of prolonging particle circulation time by preventing phagocytosis. A significant breakthrough came in 1994 when it was discovered that coating PLGA particles with the synthetic polymer polyethylene glycol, or PEG, could significantly reduce the number particles captured by the liver after injection[115]. Ever since, PEG coatings have been used to enhance circulation time and in vivo stability of particles, proteins and therapeutic drugs[116]. The effect of prolonged circulation due to PEGylation can be explained by the observation that the PEG corona reduces the adsorption of opsonins, a natural marker protein for macrophages. The PEG polymer length influences circulation time to a similar extent as particle size, with a long (10 kDa) PEG chain and small particle size (20nm) resulting in a half life greater than 48 hours[117]. Synthetic particles are still no match to red blood cells (RBCs) when it comes to circulation time. Once RBCs enter the blood stream, they may circulate up to 120 days before they are engulfed by macrophages. The unique features of RBCs have been translated to nanoparticles via a top-down approach, in which the membranes of RBCs were used to coat PLGA nanoparticles[118]. These RBCcoated nanoparticles showed a marked increase in circulation time compared to bare nanoparticles and even outlasted PEGylated nanoparticles. A key protein in preventing engulfment of RBCs by phagocytes has been identified as CD47, which acts as a “marker of self”[119]. Discher and coworkers showed that CD47 could prevent phagocytosis of opsonized microbeads, but only at a low surface density[120]. Recently, a minimal CD47 derived “self” peptide was designed and tested for inhibition of phagocytosis, which showed that this peptide was equally effective as the whole length protein[121]. For biomimetic intercellular signaling, the identification of surface ligands that prevent or slow down clearance of particles from the bloodstream is of interest because biomimetic intercellular signaling can only occur with particles that are not engulfed. 29 5. Artificial antigen-presenting cells Immunotherapy through DC vaccination or adoptive cell transfer, holds a great therapeutic potential for diseases that can be controlled using the body’s own immune system, such as cancer or autoimmune diseases. Cell-based approaches to elicit the desired cellular and humoral immune responses are often hampered to by the complex and unpredictable nature of the immune system. As touched upon previously, a promising alternative to cellular-based immunotherapy has been the development of acellular, artificial antigen-presenting cells (aAPCs), of which the exact composition can be tightly controlled. Artificial APCs have found their way in vaccination and ACT. In this chapter, we will discuss the different acellular aAPC scaffolds reported in the literature thus far to see what has driven scaffold design for aAPCs. 5.1 Basic components of an aAPC Antigen presentation by natural APCs can lead to variety of T cell responses, depending on which signals are transmitted. Therefore, control over the signals incorporated into artificial antigenpresenting cells improves control over the therapeutic outcome[122]. The information that is transmitted by an APC to activate, expand and differentiate a naïve T cell is classically divided into three signals, as illustrated in figure 22 and discussed in chapter 2. Figure 22. Schematic of signal classes presented by an antigen–presenting cell (APC). Three signals are essential for optimal T cell stimulation: 1. Recognition signals that ligate the T cell antigen receptor, pMHC complexes or antibodies cross-linking the T cell receptor (TCR), 2. Costimulatory molecules of the CD80/86 or TNF family and adhesive molecules that strengthen interactions between cells, 3. Cytokines secreted by APC or other immune cells that bind to receptors on the T cell surface[5]. The first signal, recognition, takes place when a T cell receptor (TCR) on a T cell recognizes a peptideMHC (pMHC) complex on an APC surface. For artificial antigen presentation, either an pMHC class I (for expanding CD4+ T cells) or pMHC II (for expanding CD8+ T cells) can be used as recognition signal. Often, an MHC I/II non-specific antibody, anti-CD3, is used as an alternative recognition signal on surface of aAPCs. The use of an antibody as opposed to the more biosimilar pMHC I/II should come 30 as no surprise; antibodies are more easily produced in large quantities and only one aAPC is required for the activation and expansion of a diverse repertoire of T cells. Costimulation through the interaction of CD80/86 receptors on the APC and CD28 on T cells is known to enhance the strength of the antigen-specific T cell response. Many aAPCs therefore present either CD80/86 or anti-CD28 on their surface as a second signal, although stimulation with aCD28 may only lead to T cell proliferation but not differentiation[123]. In addition to costimulatory ligands, adhesive interactions though ICAM-1 on the APC surface with LFA-1 on T cells may serve to enhance affinity and prolong APC:T cell interaction. As such, anti-LFA-1 has been used in artificial APC systems[124]. Lastly, an important factor in the rapid expansion and differentiation of T cells comes from cytokines, which are either released by the APC or by neighboring activated T cells. Cytokines are extensively used for the ex vivo culture of T cells (in adoptive transfer for example) or for direct in vivo administration as a form of immunotherapy. Cytokine release has only recently been mimicked in local delivery strategies, mainly in biodegradable PLGA particles[104] or anchored to liposomes through an Fc-fragment[125]. It is perhaps the modular, systematic description of T cell stimulation that has attracted (bio)chemical engineers to the field of artificial antigen presentation[5,6,59]. The type of T cell response can be precisely tuned, depending on the signals provided by the aAPC. Controlled display of information to T cells does not only help to increase our fundamental understanding of the nature of T cell activation, but can eventually also lead to well defined immunotherapies. 5.2 Scaffolds used for artificial antigen presentation Ever since they were introduced by Matthew Mescher in 1978, a wide variety of scaffolds have been reported for the construction of aAPCs[126]. Several reviews on the use of aAPCs in T cell activation can be found[5,127,128], of which the review by Steenblock et al. is the most Figure 23. Schematic representations of four types of acellular aAPC. Particles can be coupled with comprehensive and recognition, costimulatory or adhesion molecules by different binding schemes. Examples of configurations are shown here[5]. lists a table of the different scaffold that are used for the construction of aAPCs. An updated overview of aAPC scaffolds can be found in table 1 at the end of this chapter and the most common scaffold classes are shown in figure 23. 31 5.2.1 Liposomes Liposomes are spherical vesicles which can be prepared from amphiphilic lipid molecules such as phosphatidylcholine or cholesterol, both present in natural membranes. Their biocompatibility and ease of preparation has led many researchers to study the use of liposomes as delivery vehicles for therapeutic and diagnostic purposes early on[129,130]. Liposomes self-assemble under aqueous conditions and because of the low molecular weight of most lipids, their membrane remains fluid which allows for mechanical resizing to create vesicles from 60nm to 1 µm. Mescher and coworkers studied the use of liposomes as potential artificial antigen-presenting cells as early as 1978[126]. These crude aAPCs, consisting of purified HLA-A and HLA-B antigens reconstituted in phospholipid liposomes, were one of the first systems used to study T cell activation on a molecular level and were used to show that T cell activation is dependent on the antigen density on the liposome surface. More than two decades later, pMHC II decorated liposomal nanoparticles were found to be able to bind MHC II restricted T cells and induce interfacial pMHC:TCR clustering, demonstrating the potential of signal clustering on the aAPC[124]. Signal clustering was further explored with the use of neutravidin "rafts" comprised of pMHC complexes, anti-CD28 and anti-LFA-1 on liposomes (see figure 24)[131,132]. These preclustered, liposomal aAPCs were compared with commercially available Dynabeads® (discussed in section 5.2.3) for their ability to expand T cells. The results from this experiment showed that these aAPCs were similarly effective as the commercial microbeads, but preferentially expanded CD8+ over CD4+ T cells[132]. Recently, the pioneering work by Mescher was revisited when Ding et al. isolated lipid rafts containing pMHC complexes from DCs and reconstituted them in 200-300 nm-sized liposomes[133]. These so-called RAFTsomes effectively expand T cells in vitro and elicit both cellular and humoral immune responses in mice. Figure 24. Schematic of an artificial APC depicting a section of the lipid bilayer. GM-1 ganglioside incorporated into the bilayer binds one molecule of choleratoxin β (CTB) biotin. In turn, one molecule of neutravidin anchors the biotinylated anti-CD28, the pMHC complex and biotinanti-LFA-1 to the aAPC through the CTBbiotin molecule[170]. Liposomes share interesting properties with natural APCs, such as membrane fluidity and biocompatibility. Unfortunately, the trade-off for the fluid nature of liposomal membranes is their poor stability, which greatly hampers their use as an aAPC[134]. To overcome this problem, other, more stable scaffolds have been developed. 5.2.2 Supported lipid bilayers Supported lipid bilayers (SLB) on a planar surface are often used to study the molecular details of the immune synapse, as discussed in chapter 2. However, supported lipid bilayers can also be constructed on spherical surfaces, in which lipids are coated around a solid bead[135,136]. Such spherical SLBs allow for a lateral movement of surface bound ligands, while their stability is increased compared to liposomes[93]. Cell-sized, spherical SLBs were prepared by coating solid microparticles 32 with a lipid layer that anchored pMHC I. These SLB-based aAPCs were not able to expand CTLs from naïve T cells, but they were capable of restimulating previously primed CTLs[137]. Micrometer sized, artificial APCs prepared by coating silica microparticles with melanoma cell-lysates showed no significant responses in stage IV melanoma patients, possibly due to lack of exogenous activating cytokines[138]. 5.2.3 Polystyrene beads Uniform, solid polystyrene beads can easily be prepared in different sizes from the polymerization of styrene via controlled emulsion polymerization. Although such polystyrene (PS) beads lack the fluid outer membrane and aqueous inner core of liposomes, they are easily handled and are readily surface modified through non-covalent interactions with the particle surface. These beads have been used as aAPCs to study the effects of, amongst other things, particle size and ligand density on T cell stimulation[64,139–147]. For example, it was shown that in the range of 1-5 µm, 4-5 µm PS particles coated with pMHC I were optimally suited for CTL activation. Below 4 µm, activation of CTLs decreased rapidly, an effect which could not be compensated by the addition of more particles[64]. These findings suggest that a large, continuous surface contact between an aAPC and a T cell is required for optimal signal transduction. Additionally, it was found that stimulation by surface bound CD86 alone did not result in activation of T cells, but that this required the presence of additional anti-TCR antibodies, and that the effectiveness of CD86 costimulation was dependant on its surface density[139]. Although signal 1 and 2 on an aAPC surface is enough to activate T cells, exogenous addition of cytokines is required to ensure proliferation and effector differentiation after initial activation[35,148]. In a move towards the clinical use of aAPCs, PS beads coated with (HLA)-A2/pIL-13Rα2345-354 tetramers, anti-CD28 and CD83 were used to expand antigen specific CTLs from peripheral blood derived mononuclear cells (PMBCs) obtained from healthy donors. The ex vivo expanded CTLs showed lytic activity HLA-A2+ glioma cells that expressed IL-13Rα2, whereas no lytic activity was observed for HLA-A2- that did not express IL-13Rα2[143]. More recently, PS-based aAPCs were used to generate a melanoma-specific CTLs response in vivo. Artificial APCs consisting of H-2K(b)/TRP2 tetramers, anti-CD28 and anti-4-1BB coupled to 5 µm PS beads, were injected intravenously and subcutaneously into naïve or antigen-primed mice which resulted in a vigorous CTL response[147]. Interestingly, no harmful side-effects such as embolism was reported in these studies. A special class of PS-bead based aAPCs incorporates an iron oxide core within a PS coating and an outer layer of reactive tosyl-, or epoxide groups. These beads, commercially sold under the Dynabead® brand name, allow for the attachment of a variety of ligands, and the iron oxide core enables easy magnetic removal of the aAPCs from the culture medium, making this system optimally suited for ACT based therapies. These beads are often used to non-specifically expand T cells from a population of PBMCs, although they can be used for a wide range of applications[149]. Magnetic aAPCs have been equipped with aCD3[123,150–153], MHC tetramers[154] or HLA-Ig dimers[155–161] in combination with surface bound or soluble costimulatory molecules in the context of T cell stimulation. Genetically produced HLA-Ig constructs confer mechanical stability to the HLA:TCR interaction, which increases T cell binding affinity[155,156]. HLA-Ig coated magnetic aAPCs, in combination with exogenously delivered IL-2, effectively expanded MART-1 specific CTLs from a population of CD8+ PBMCs, generating up to 109 cells within two months[155]. 33 Although both the magnetic and the regular PS beads are versatile and easy to handle platforms for T cell activation, their material properties limit the extent to which biomimetic T cell stimulation can be achieved. Both paracrine release of cytokines and dynamic redistribution of ligands is not possible with this system. Moreover, the method of attachment to these particles can interfere with the proper orientation of the displayed ligands, thereby hampering signal transduction to T cells[162]. 5.2.4 Biodegradable particles When transient stimulation by aAPCs is required, for example in active vaccination strategies or other in vivo applications, biodegradable polymeric scaffolds may be superior over nonbiodegradable scaffolds. In addition, biodegradable scaffolds are capable of encapsulating cytokines, which can be released in a paracrine manner, allowing for a local, sustained cytokine-rich environment. A team led by Terek Fahmy has focused on the production of such biodegradable aAPCs, based on PLGA polymers[65,104,163]. This copolymer can be made into spherical particles with well defined sizes and the rate of degradation can be finely tuned by adjusting the PLA/PGA ratio. To functionalize these polymers, palmitate-conjugated streptavidin is mixed in with the polymer during the fabrication process, which ends up on the outside of the particle after production. This streptavidin moiety can then be modified to display any biotinylated biomolecule of interest. In a comparative study, either micro- or nano-sized PLGA particles were modified to display pMHC I dimers and anti-CD28 and consequently used to stimulate CD8+ T cells[65]. Micron-sized aAPCs outperformed both nano-sized aAPCs and soluble pMHC I dimers, as measured by cytokine secretion. In the same study, it was shown that costimulation through CD28 significantly enhances T cell proliferation and cytokine secretion and that the overall T cells count is increased when aAPCs display pMHC I dimers instead of aCD3 as a recognition signal. Finally, enhanced expansion of T cells was observed when IL-2 was encapsulated in the polymer matrix. These biodegradable aAPC were shown to outperform autologous APCs for the expansion of antigen-specific T cells in vitro[164]. A possible limitation to these biodegradable systems is their intrinsic instability, which could limit the time that ligands stay at the aAPC surface. 5.2.5 Other scaffolds Next to the scaffolds mentioned previously, there numerous additional materials that could serve as a scaffold for the multimeric display of T cell activating ligands. Some interesting materials that have been tested as scaffolds for artificial antigen presentation include carbon nanotubes[165], polyisocyanides[166] and mannose nanofibers[167]. The development of novel scaffolds is of interest to the field of artificial antigen presentation, since each material gives us more insight into the chemical and mechanical requirements for optimal T cell activation. 34 Table 1. Artificial antigen-presenting platforms reported in the literature (adapted from[5]) Platform Liposomes Supported lipid bilayers Polystyrene beads Size (common) 60-1000 nm (100 nm) 5 µm Signal 1 Signal 2 HLA-A/HLA-B, pMHC Anti-CD28, antiII, HLA-DR4, antiLFA-1 CD3 pMHC I - IL-2/ IL-15 1-10 µm (5 µm) Tumor cell membrane, pMHC I, anti-CD3, HLA-A, H2Kb-Ig dimers, MHC II dimers, HLA-A2 tetramers Anti-CD3, HLA tetramers, HLA-A2Ig dimers Tumor cell membrane, antiCD28, anti-CD2, 4-1BB ligand, anti-4-1BB, CD83 Anti-CD28, anti4-1BB, anti-FasIgM, CD80-Ig, CD86-Ig Anti-CD28 Magnetic polystyrene beads 4.5 µm PLGA beads 130 nm-8 µm (7 µm) Carbon nanotubes 500 nm (bundles) Polyisocyanides 2 x 120 nm Anti-CD3 - Self-asembled fibers 40nm x ~3µm - - Anti-CD3, MHC-KB dimers, HLA-A2-Ig dimers pMHC I Signal 3 Ligand attachment Hydrophobic, Avidin-Biotin In vitro stimulation Murine splenocytes, Human PBMCs In vivo Ref. use [124,126,131,132,168] Active (humans) Con A Hydrophobic Murine splenocytes - [137] IL-2, IL-4, IL-7, IL-12 Adsorption, avidin-biotin, covalent non selective Murine splenocytes, Human PBMCs [64,141–146] IL-2, IL-7, TCGF Covalent, nonselective Murine splenocytes, Human PBMCs Active (mice), adoptive (mice and humans) Adoptive (mice and humans) Active (mice) IL-2 Avidin-biotin (paracrine) Murine splenocytes, Human PBMCs [123,150–160] [65,104,163,164] Avidin-biotin Ag specific murine CD8+ lymphocytes - [165] - Avidin-biotin Human PBMCs - [166] Con A Electrostatic Jurkat cells - [167] Anti-CD28 (soluble) Nb. Signals 1 and 2 are surface bound unless state otherwise. Signal 3 is exogenously added unless stated otherwise. 6. Conclusions Work with dendritic cell-based vaccines has proven that clinically relevant results can be obtained by harnessing the power of the immune system to fight cancer. However, such APC-based therapies are often laborious and costly, limiting their widespread application. This, in addition to the unpredictable outcome of DC manipulation, has led to the development of modular, tunable systems for T cell activation known as artificial antigen-presenting cells. As the name suggests, artificial antigen-presenting cells should mimic antigen-presentation by professional antigen-presenting cells. The activation of naïve T cells by DCs (a professional APC) has been studied extensively, from the molecular to the systemic level. Naïve T cells are primarily activated in the lymph nodes, where they scan their surroundings at high speeds until they encounter their cognate pMHC complex on the surface of a DC. Only a few (<10) pMHC complexes are required for the T cell to bind, but once the T cell is bound, it can stay bound for hours. Initial contact leads to the formation of an immunological synapse (IS) between the DC and the T cell. The macromolecular structure of the IS influences T cell activation, through the formation of multiple, spatially separated ligand-receptor interactions. Although the precise role of the overall IS structure on T cell activation is not known, it seems to be essential that TCRs are preclustered, preferably together with costimulatory receptors such as CD28, on the T cell surface. In addition, stimulatory cytokines, such as IL-2, need to be present to assure proliferation of the activated T cells. The stimulatory capabilities of an APC have successfully been mimicked by artificial antigenpresenting platforms, varying from liposomal to solid polymeric constructs. Especially in vitro, such systems have proven their value as modular platforms for the stimulation of T cells. However, some essential characteristics of natural T cell stimulation, such as dynamic remodeling of surface bound ligands or the paracrine delivery of cytokines, remain hard to mimic. Another point of concern related to the possible therapeutic use of aAPCs is related to the large size (5-10 µm) that seems to be required for optimal T cell stimulation. Although ex vivo cultured T cells put no restriction on aAPC size, the intravenous delivery of particles larger than a few micrometers may lead to embolism. For this reason, it may be of interest to develop nano-sized aAPCs for the in vivo stimulation of T cells[163]. The choice of the right material for the construction of an aAPC is definitely not trivial, as basic scaffold material properties partially determine how signals are presented by the aAPC. An ideal aAPC is structurally stable and allows for a well defined ligand decoration, while preferably being biocompatible/biodegradable as well. Since the scaffolds reported thus far have primarily been selected based on availability of the scaffold, development of novel scaffolds could open up the possibility to systematically study unexplored biomimetic factors such as shape or surface fluidity. For the development of new artificial antigen-presenting cells, the ultimate application of such aAPCs should be kept in mind. When used as a model system for the stimulation of T cells in vitro, the choice of scaffold is almost unrestricted. However, when such aAPCs are expected to be used in active vaccination strategies, the scaffold must be biocompatible and small enough to prevent embolism. 36 Artificial antigen-presenting cells can provide a powerful tool for unraveling the molecular details of T cell activation and show potential use for cancer immunotherapy. The artificial antigen-presenting platforms presented in the literature up to date show that it is possible to stimulate T cells in a biomimetic way using simplified constructs. Although not all requirements for T cell activation, and particularly differentiation, are known, these constructs provide a solid basis from which new artificial antigen-presenting cells can be constructed. 37 II. Summary The immune system is perfectly adapted to protect against disease by constantly monitoring the body for anything which is not the body’s own. Any unfamiliar agent, either a virus or a misfolded protein, may lead to an immune response. Key players in mediating the immune response are dendritic cells (DCs), a professional type of antigen-presenting cell (APC). DCs capture, process and present compounds that are derived from undesired agents (antigens) via MHC/antigen complexes on their cell surface to activate T cells, which in turn track down the antigen source and clear it from the body. Through their function as antigen-presenting cells, DCs are vital mediators in the development of cancer immunity, and it is this finding that has led researchers to seek ways of manipulating the immune system in the context of cancer immunotherapy. Broadly, two approaches to cancer immunotherapy exist; direct DC manipulation in order to promote DC-mediated antigen presentation or adoptive transfer of ex vivo cultured, DC-activated tumoricidal T cells. Both approaches rely on antigen-presentation by natural DCs, which is still problematic due to the lack of knowledge on DC:T cell communication and the difficulty of culturing DCs. For a better understanding of T cell activation, artificial antigen-presenting cells (aAPCs) have been developed. These simplified DC mimics usually incorporate the basic elements required for T cell activation on a material scaffold and have proven useful for the production of large amounts of activated T cells. APCs deliver three kind of signals to T cells; activation via the recognition of an MHC complex by a T cell receptor (TCR), survival though additional interactions with receptors on the T cell surface, and differentiation via soluble cytokines released by the APC. During T cell activation, a large interface is formed between the APC and the T cell, known as an immunological synapse (IS). From studies where the APC is mimicked by a supported lipid bilayer (SLB), we know that only a few MHC:TCR interactions are enough for the formation of an IS and that the structural orientation of the activation and survival signals strongly influence the outcome of overall T cell activation. Although there is little variation in the ligands that are incorporated on the surface of aAPCs, various completely different material scaffolds have been tested over the years, with different success rates. Liposomes were classically used, due to their ease of production, but were abandoned because of stability issues. A commonly used scaffold nowadays is the solid polystyrene bead, which has proven optimal for T cell activation at sizes comparable to cells. For potential in vivo use, biodegradable polymeric aAPCs have been developed, which in addition to activation and survival signals, also release cytokines during their degradation. The physical properties of the particles that are used for the production of aAPCs are of importance to biomimetic antigen presentation. It is well known that particle size affects their interaction with cells, but less is known about particle shape or the lateral mobility of ligands on a particle surface. Advances in material design allow for the production of ever more complex materials on a nano- and micrometer scale, the scale at which cells operate. Extrapolation of the results from cellular interaction with such novel materials to T cell activation, suggests that such novel materials could be used as scaffolds for the production of aAPCs. 38 III. Samenvatting Ons immuun system heeft zich ontwikkeld tot een geavanceerd afweermechanisme tegen infecties van buitenaf (virussen, bacteriën), maar ook verkeerd gevouwen lichaamseigen eiwitten of abnormaal groeiende cellen. Gespecialiseerde cellen van het immuunsysteem, dendritische cellen (DCs), houden zich bezig met het vangen van kwaadaardige deeltjes in het lichaam. De kwaadaardige deeltjes worden verwerkt tot kleine stukjes, ook wel antigenen genoemd, die vervolgens middels receptoren op het oppervlak van DCs aan T cellen worden gepresenteerd. De functie van een T cel is als een speurhond; nadat een DC een antigen aan een T cel heeft gepresenteerd, gaat de T cel op zoek naar kwaadaardige deeltjes waar het antigen vandaan kwam en ruimt deze op. Het lichaam past deze vorm van cellulaire immuniteit ook toe bij het opruimen van kankercellen, alhoewel deze kankercellen zich niet altijd even makkelijk laten vinden. Dit heeft onderzoekers gemotiveerd om het immuunsysteem een handje te helpen in het bestrijden van kanker. Er zijn twee verschillende manieren om dit te doen; ervoor zorgen dat de DCs in het lichaam extra antigen tot hun beschikking hebben om te presenteren aan T cellen, of T cellen buiten het lichaam opkweken onder optimale omstandigheden en ze terugplaatsen. Voor beide methodes worden DCs gebruikt, maar door de complexiteit van de interacties tussen DCs en T cellen levert dit niet altijd functionele T cellen op. Om de complexiteit die DCs met zich meebrengen te omzeilen, kan er ook gebruik gemaakt worden van kunstmatige DCs. Kunstmatige DCs bestaan vaak uit kleine (micro- of nano)deeltjes, die de minimale hoeveel informatie bevatten om T cellen te kunnen activeren. Zulke kunstmatige DCs zijn met succes gebruikt voor het opkweken van kanker-opruimende T cellen. Dendritische cellen dragen drie verschillende soorten signalen over aan T cellen; activeren, overleven en differentiëren. De signalen voor activeren en differentiëren worden direct overgedragen via het cel oppervlak en het signaal voor differentiëren wordt indirect uitgescheiden door DCs. Via verschillende experimenten weten we dat DCs en T cellen tijdens signaaloverdracht een synaps vormen, en dat binnen deze synaps de signalen voor activeren en overleven zich ordenen in een uniek patroon. Uit deze experimenten blijkt dus dat het belangrijk is dat de signalen op het oppervlak van een (kunstmatige) DC vrij kunnen bewegen of in de juiste structuur moeten zitten. Kunstmatige DCs bestaan in verschillende soorten. Wat ze gemeen hebben is dat ze een over activerend en overlevingssignaal beschikken en ze daarmee, met behulp van een toegevoegd differentiatiesignaal, T cellen kunnen activeren. De eerste kunstmatige DCs werden gemaakt van liposomen, waarop oppervlak-gebonden signalen vrijelijk kunnen bewegen. Helaas bleken deze deeltjes niet stabiel genoeg om gebruikt te worden als kunstmatige DCs. Een stabieler systeem voor de productie van kunstmatige DCs is gebaseerd op rubberen bolletjes, waarbij bolletjes ter grootte van cellen het beste waren in het activeren van T cellen. Een recenter systeem is gebaseerd op afbreekbare polymeren, die niet alleen over activerend en overlevingssignaal beschikken, maar ook een differentiatiesignaal kan afgeven tijdens het afbraakproces. Het materiaal waaruit een kunstmatige DC bestaat, heeft een grote invloed op de manier waarop de verschillende signalen gepresenteerd worden. Nieuwe ontwikkelingen in de productie van micro- en nanodeeltjes maken het mogelijk om niet alleen naar de effecten van grootte, maar ook bijvoorbeeld vorm en stijfheid op T cel activatie te kijken. Vanuit andere studies met cellen is al het een en ander 39 bekend over de invloed van deze “nieuwe” eigenschappen van micro- en nanodeeltjes, maar binnen het gebied van T cel activatie met kunstmatige DCs valt nog een hoop te ontdekken. 40 IV. Bibliography [1] M. S. Diamond, M. Kinder, H. Matsushita, M. Mashayekhi, G. P. Dunn, J. M. Archambault, H. Lee, C. D. Arthur, J. M. White, U. Kalinke, et al., The Journal of experimental medicine 2011, 208, 1989–2003. [2] M. B. Fuertes, A. K. Kacha, J. Kline, S.-R. Woo, D. M. Kranz, K. M. Murphy, T. F. Gajewski, The Journal of experimental medicine 2011, 208, 2005–16. [3] C. G. Figdor, I. J. M. de Vries, W. J. Lesterhuis, C. J. M. Melief, Nature medicine 2004, 10, 475– 80. [4] D. H. Schuurhuis, P. Verdijk, G. Schreibelt, E. H. J. G. Aarntzen, N. Scharenborg, A. de Boer, M. W. M. M. van de Rakt, M. Kerkhoff, M.-J. P. Gerritsen, F. Eijckeler, et al., Cancer research 2009, 69, 2927–34. [5] E. R. Steenblock, S. H. Wrzesinski, R. a Flavell, T. M. Fahmy, Expert opinion on biological therapy 2009, 9, 451–64. [6] S. C. Balmert, S. R. Little, Advanced materials (Deerfield Beach, Fla.) 2012, 24, 3757–78. [7] K. Murphy, Janeway’s Immunobiology, 8th Edition, Garland Science, 2012. [8] T. T. Doan, R. Melvold, C. Waltenbaugh, Concise Medical Immunology, Lippincott Williams & Wilkins, 2005. [9] P. Guermonprez, J. Valladeau, L. Zitvogel, C. Théry, S. Amigorena, Annual review of immunology 2002, 20, 621–67. [10] K. Shortman, Y.-J. Liu, Nature reviews. Immunology 2002, 2, 151–61. [11] G. J. Randolph, V. Angeli, M. a Swartz, Nature reviews. Immunology 2005, 5, 617–28. [12] J. E. Smith-Garvin, G. a Koretzky, M. S. Jordan, Annual review of immunology 2009, 27, 591– 619. [13] R. M. Steinman, D. Hawiger, M. C. Nussenzweig, Annual review of immunology 2003, 21, 685– 711. [14] A. W. Thomson, H. R. Turnquist, A. F. Zahorchak, G. Raimondi, Transplantation 2009, 87, S86– 90. [15] A. Grakoui, Science 1999, 285, 221–227. [16] M. L. Dustin, D. Depoil, Nature reviews. Immunology 2011, 11, 672–84. [17] M. J. Miller, O. Safrina, I. Parker, M. D. Cahalan, The Journal of experimental medicine 2004, 200, 847–56. 41 [18] M. J. Miller, A. S. Hejazi, S. H. Wei, M. D. Cahalan, I. Parker, Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 998–1003. [19] D. J. Irvine, M. a Purbhoo, M. Krogsgaard, M. M. Davis, Nature 2002, 419, 845–9. [20] M. a Purbhoo, D. J. Irvine, J. B. Huppa, M. M. Davis, Nature immunology 2004, 5, 524–30. [21] A. Chisholm, A. Zahler, J. Sisson, V. Ambros, Nature 1998, 340, 764–766. [22] D. R. Fooksman, S. Vardhana, G. Vasiliver-Shamis, J. Liese, D. a Blair, J. Waite, C. Sacristán, G. D. Victora, A. Zanin-Zhorov, M. L. Dustin, Annual review of immunology 2010, 28, 79–105. [23] L. J. Edwards, V. I. Zarnitsyna, J. D. Hood, B. D. Evavold, C. Zhu, Frontiers in immunology 2012, 3, 86. [24] J. Xie, J. B. Huppa, E. W. Newell, J. Huang, P. J. R. Ebert, Q.-J. Li, M. M. Davis, Nature immunology 2012, 13, 674–80. [25] P. Friedl, A. T. den Boer, M. Gunzer, Nature reviews. Immunology 2005, 5, 532–45. [26] B. N. Manz, J. T. Groves, Nature reviews. Molecular cell biology 2010, 11, 342–52. [27] Y. Kaizuka, A. D. Douglass, R. Varma, M. L. Dustin, R. D. Vale, Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 20296–301. [28] M. J. P. Biggs, M. C. Milone, L. C. Santos, a Gondarenko, S. J. Wind, Journal of the Royal Society, Interface / the Royal Society 2011, 8, 1462–71. [29] J. Doh, D. J. Irvine, Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 5700–5. [30] M. L. Dustin, J. T. Groves, Annual review of biophysics 2012, 41, 543–56. [31] J. J. Illingworth, P. Anton van der Merwe, Immunology 2012, 135, 198–206. [32] K. Palucka, J. Banchereau, Nature reviews. Cancer 2012, 12, 265–77. [33] D. L. Porter, B. L. Levine, M. Kalos, A. Bagg, C. H. June, The New England journal of medicine 2011, 365, 725–33. [34] I. Mellman, G. Coukos, G. Dranoff, Nature 2011, 480, 480–9. [35] M. F. Mescher, J. M. Curtsinger, P. Agarwal, K. a Casey, M. Gerner, C. D. Hammerbeck, F. Popescu, Z. Xiao, Immunological reviews 2006, 211, 81–92. [36] P. a Antony, C. a Piccirillo, A. Akpinarli, S. E. Finkelstein, P. J. Speiss, D. R. Surman, D. C. Palmer, C.-C. Chan, C. a Klebanoff, W. W. Overwijk, et al., Journal of immunology (Baltimore, Md. : 1950) 2005, 174, 2591–601. [37] E. H. J. G. Aarntzen, I. J. M. De Vries, W. J. Lesterhuis, D. Schuurhuis, J. F. M. Jacobs, K. Bol, G. Schreibelt, R. Mus, J. H. W. De Wilt, J. B. a G. Haanen, et al., Cancer research 2013, 73, 19–29. 42 [38] L. Bonifaz, D. Bonnyay, K. Mahnke, M. Rivera, M. C. Nussenzweig, R. M. Steinman, Journal of Experimental Medicine 2002, 196, 1627–1638. [39] Z. Zhang, S. Tongchusak, Y. Mizukami, Y. J. Kang, T. Ioji, M. Touma, B. Reinhold, D. B. Keskin, E. L. Reinherz, T. Sasada, Biomaterials 2011, 32, 3666–78. [40] T. Yoshikawa, N. Okada, A. Oda, K. Matsuo, K. Matsuo, H. Kayamuro, Y. Ishii, T. Yoshinaga, T. Akagi, M. Akashi, et al., Vaccine 2008, 26, 1303–13. [41] S. T. Reddy, A. J. van der Vlies, E. Simeoni, V. Angeli, G. J. Randolph, C. P. O’Neil, L. K. Lee, M. a Swartz, J. a Hubbell, Nature biotechnology 2007, 25, 1159–64. [42] N.-H. Cho, T.-C. Cheong, J. H. Min, J. H. Wu, S. J. Lee, D. Kim, J.-S. Yang, S. Kim, Y. K. Kim, S.-Y. Seong, Nature nanotechnology 2011, 6, 675–82. [43] S. L. Demento, A. L. Siefert, A. Bandyopadhyay, F. a Sharp, T. M. Fahmy, Trends in biotechnology 2011, 29, 294–306. [44] N. P. Restifo, M. E. Dudley, S. a Rosenberg, Nature reviews. Immunology 2012, 12, 269–81. [45] S. a Rosenberg, J. C. Yang, R. M. Sherry, U. S. Kammula, M. S. Hughes, G. Q. Phan, D. E. Citrin, N. P. Restifo, P. F. Robbins, J. R. Wunderlich, et al., Clinical cancer research : an official journal of the American Association for Cancer Research 2011, 17, 4550–7. [46] M. L. Disis, H. Bernhard, E. M. Jaffee, Lancet 2009, 373, 673–83. [47] C. H. June, B. R. Blazar, J. L. Riley, Nature reviews. Immunology 2009, 9, 704–16. [48] L.-X. Wang, S. Shu, G. E. Plautz, Cancer research 2005, 65, 9547–54. [49] A. Mackensen, N. Meidenbauer, S. Vogl, M. Laumer, J. Berger, R. Andreesen, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006, 24, 5060–9. [50] C. Yee, J. a Thompson, D. Byrd, S. R. Riddell, P. Roche, E. Celis, P. D. Greenberg, Proceedings of the National Academy of Sciences of the United States of America 2002, 99, 16168–73. [51] C. M. Paulos, M. M. Suhoski, G. Plesa, T. Jiang, S. Basu, T. N. Golovina, S. Jiang, N. a Aqui, D. J. Powell, B. L. Levine, et al., Immunologic research 2008, 42, 182–96. [52] L. Galluzzi, E. Vacchelli, A. Eggermont, W. H. Fridman, J. Galon, C. Sautès-Fridman, E. Tartour, L. Zitvogel, G. Kroemer, Oncoimmunology 2012, 1, 306–315. [53] S. Mitragotri, J. Lahann, Nature materials 2009, 8, 15–23. [54] J.-W. Yoo, D. J. Irvine, D. E. Discher, S. Mitragotri, Nature reviews. Drug discovery 2011, 10, 521–35. [55] K. D. Young, Microbiology and molecular biology reviews : MMBR 2006, 70, 660–703. [56] R. a Petros, J. M. DeSimone, Nature reviews. Drug discovery 2010, 9, 615–27. 43 [57] J. a Hubbell, S. N. Thomas, M. a Swartz, Nature 2009, 462, 449–60. [58] M. a Swartz, S. Hirosue, J. a Hubbell, Science translational medicine 2012, 4, 148rv9. [59] J. J. Moon, B. Huang, D. J. Irvine, Advanced materials (Deerfield Beach, Fla.) 2012, 24, 3724– 46. [60] S. D. Conner, S. L. Schmid, Nature 2003, 422, 37–44. [61] S. E. a Gratton, P. a Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier, J. M. DeSimone, Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 11613–8. [62] J. a Champion, A. Walker, S. Mitragotri, Pharmaceutical research 2008, 25, 1815–21. [63] S. S. Yu, C. M. Lau, S. N. Thomas, W. G. Jerome, D. J. Maron, J. H. Dickerson, J. a Hubbell, T. D. Giorgio, International journal of nanomedicine 2012, 7, 799–813. [64] M. F. Mescher, Journal of immunology (Baltimore, Md. : 1950) 1992, 149, 2402–5. [65] E. R. Steenblock, T. M. Fahmy, Molecular therapy : the journal of the American Society of Gene Therapy 2008, 16, 765–72. [66] N. Bertrand, J.-C. Leroux, Journal of controlled release : official journal of the Controlled Release Society 2012, 161, 152–63. [67] D. Kohane, Biotechnology and bioengineering 2007, 96, 203–209. [68] T. J. Merkel, S. W. Jones, K. P. Herlihy, F. R. Kersey, A. R. Shields, M. Napier, J. C. Luft, H. Wu, W. C. Zamboni, A. Z. Wang, et al., Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 586–91. [69] K. Y. Dane, C. Nembrini, A. a Tomei, J. K. Eby, C. P. O’Neil, D. Velluto, M. a Swartz, L. Inverardi, J. a Hubbell, Journal of controlled release : official journal of the Controlled Release Society 2011, 156, 154–60. [70] A. Stano, C. Nembrini, M. a Swartz, J. a Hubbell, E. Simeoni, Vaccine 2012, 30, 7541–6. [71] A. Stano, E. a Scott, K. Y. Dane, M. a Swartz, J. a Hubbell, Biomaterials 2013, 34, 4339–46. [72] J. J. Moon, H. Suh, A. V Li, C. F. Ockenhouse, A. Yadava, D. J. Irvine, Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 1080–5. [73] J. a Champion, Y. K. Katare, S. Mitragotri, Journal of controlled release : official journal of the Controlled Release Society 2007, 121, 3–9. [74] N. Daum, C. Tscheka, A. Neumeyer, M. Schneider, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology 2012, 4, 52–65. [75] G. Sharma, D. T. Valenta, Y. Altman, S. Harvey, H. Xie, S. Mitragotri, J. W. Smith, Journal of controlled release : official journal of the Controlled Release Society 2010, 147, 408–12. 44 [76] J. a Champion, S. Mitragotri, Pharmaceutical research 2009, 26, 244–9. [77] J.-W. Yoo, S. Mitragotri, Proceedings of the National Academy of Sciences 2010, 107, 11205– 11210. [78] J. a Champion, S. Mitragotri, Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 4930–4. [79] L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy, V. Mailänder, Small (Weinheim an der Bergstrasse, Germany) 2012, 8, 2222–30. [80] S. Muro, C. Garnacho, J. a Champion, J. Leferovich, C. Gajewski, E. H. Schuchman, S. Mitragotri, V. R. Muzykantov, Molecular therapy : the journal of the American Society of Gene Therapy 2008, 16, 1450–8. [81] Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko, D. E. Discher, Nature nanotechnology 2007, 2, 249–55. [82] S. Barua, J.-W. Yoo, P. Kolhar, A. Wakankar, Y. R. Gokarn, S. Mitragotri, Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 3270–5. [83] N. Doshi, A. S. Zahr, S. Bhaskar, J. Lahann, S. Mitragotri, Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 21495–9. [84] K. a Beningo, Y. Wang, Journal of cell science 2002, 115, 849–56. [85] G. Robbins, D. Lee, J. Katz, P. Frail, Soft Matter 2010, 769–779. [86] E. Judokusumo, E. Tabdanov, S. Kumari, M. L. Dustin, L. C. Kam, Biophysical journal 2012, 102, L5–7. [87] H.-S. Han, J. D. Martin, J. Lee, D. K. Harris, D. Fukumura, R. K. Jain, M. Bawendi, Angewandte Chemie (International ed. in English) 2013, 52, 1414–9. [88] M. L. Dustin, The Journal of clinical investigation 2012, 122, 1149–55. [89] J. B. Huppa, M. Axmann, M. a Mörtelmaier, B. F. Lillemeier, E. W. Newell, M. Brameshuber, L. O. Klein, G. J. Schütz, M. M. Davis, Nature 2010, 463, 963–7. [90] L. L. Kiessling, J. E. Gestwicki, L. E. Strong, Angewandte Chemie (International ed. in English) 2006, 45, 2348–68. [91] A. Dolganiuc, World journal of gastroenterology : WJG 2011, 17, 2520–35. [92] B. M. Discher, Science 1999, 284, 1143–1146. [93] C. E. Ashley, E. C. Carnes, G. K. Phillips, D. Padilla, P. N. Durfee, P. A. Brown, T. N. Hanna, J. Liu, B. Phillips, M. B. Carter, et al., Nature materials 2011, 10, 389–97. [94] M. Porotto, F. Yi, A. Moscona, D. a LaVan, PloS one 2011, 6, e16874. 45 [95] D. a. Hammer, G. P. Robbins, J. B. Haun, J. J. Lin, W. Qi, L. a. Smith, P. Peter Ghoroghchian, M. J. Therien, F. S. Bates, Faraday Discussions 2008, 139, 129. [96] W. R. Algar, D. E. Prasuhn, M. H. Stewart, T. L. Jennings, J. B. Blanco-Canosa, P. E. Dawson, I. L. Medintz, Bioconjugate chemistry 2011, 22, 825–58. [97] M. Labowsky, T. M. Fahmy, Chemical engineering science 2012, 74, 114–123. [98] R. Langer, J. Folkman, Nature 1976, 263, 797–800. [99] G. Crotts, T. G. Park, Journal of microencapsulation 1998, 15, 699–713. [100] T. G. Park, H. Yong Lee, Y. Sung Nam, Journal of controlled release : official journal of the Controlled Release Society 1998, 55, 181–91. [101] S. N. Rothstein, S. R. Little, Journal of Materials Chemistry 2011, 21, 29. [102] L. Zhou, M. M. W. Chong, D. R. Littman, Immunity 2009, 30, 646–55. [103] M. G. Tovey, C. Lallemand, Vaccine Adjuvants, Humana Press, Totowa, NJ, 2010. [104] E. R. Steenblock, T. Fadel, M. Labowsky, J. S. Pober, T. M. Fahmy, The Journal of biological chemistry 2011, 286, 34883–92. [105] J. Park, W. Gao, R. Whiston, T. B. Strom, S. Metcalfe, T. M. Fahmy, Molecular pharmaceutics 2011, 8, 143–52. [106] H. Nakase, K. Okazaki, Y. Tabata, M. Ozeki, N. Watanabe, M. Ohana, S. Uose, K. Uchida, T. Nishi, M. Mastuura, et al., The Journal of pharmacology and experimental therapeutics 2002, 301, 59–65. [107] J. W. Koten, M. J. a. Van Luyn, J. a. Cadée, L. Brouwer, W. E. Hennink, C. Bijleveld, W. Den Otter, Cytokine 2003, 24, 57–66. [108] J. Park, S. H. Wrzesinski, E. Stern, M. Look, J. Criscione, R. Ragheb, S. M. Jay, S. L. Demento, A. Agawu, P. Licona Limon, et al., Nature materials 2012, 11, 895–905. [109] S. Jhunjhunwala, S. C. Balmert, G. Raimondi, E. Dons, E. E. Nichols, A. W. Thomson, S. R. Little, Journal of controlled release : official journal of the Controlled Release Society 2012, 159, 78– 84. [110] U. K. Kar, M. K. Srivastava, A. Andersson, F. Baratelli, M. Huang, V. a Kickhoefer, S. M. Dubinett, L. H. Rome, S. Sharma, PloS one 2011, 6, e18758. [111] X. Zhao, S. Jain, H. Benjamin Larman, S. Gonzalez, D. J. Irvine, Biomaterials 2005, 26, 5048–63. [112] S. Jhunjhunwala, G. Raimondi, A. J. Glowacki, S. J. Hall, D. Maskarinec, S. H. Thorne, A. W. Thomson, S. R. Little, Advanced materials (Deerfield Beach, Fla.) 2012, 24, 4735–8. [113] D. J. Irvine, A. N. Stachowiak, Y. Hori, Seminars in immunology 2008, 20, 137–46. 46 [114] Y. Wang, D. J. Irvine, Integrative biology : quantitative biosciences from nano to macro 2013, 5, 481–94. [115] R. Gref, Y. Minamitake, M. Peracchia, V. Trubetskoy, V. Torchilin, R. Langer, Science 1994, 263, 1600–1603. [116] K. Knop, R. Hoogenboom, D. Fischer, U. S. Schubert, Angewandte Chemie (International ed. in English) 2010, 49, 6288–308. [117] S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer, W. C. W. Chan, Nano letters 2009, 9, 1909– 15. [118] C.-M. J. Hu, L. Zhang, S. Aryal, C. Cheung, R. H. Fang, L. Zhang, Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 10980–5. [119] P. -a. Oldenborg, A. Zheleznyak, Y.-F. Fang, C. F. Lagenaur, H. D. Gresham, F. P. Lindberg, Science 2000, 288, 2051–2054. [120] R. K. Tsai, P. L. Rodriguez, D. E. Discher, Blood cells, molecules & diseases 2010, 45, 67–74. [121] P. L. Rodriguez, T. Harada, D. a. Christian, D. a. Pantano, R. K. Tsai, D. E. Discher, Science 2013, 339, 971–975. [122] J. V Kim, J.-B. Latouche, I. Rivière, M. Sadelain, Nature biotechnology 2004, 22, 403–10. [123] C. P. Broeren, G. S. Gray, B. M. Carreno, C. H. June, Journal of immunology (Baltimore, Md. : 1950) 2000, 165, 6908–14. [124] B. Prakken, M. Wauben, D. Genini, R. Samodal, J. Barnett, a Mendivil, L. Leoni, S. Albani, Nature medicine 2000, 6, 1406–10. [125] B. Kwong, S. A. Gai, J. Elkhader, K. D. Wittrup, D. J. Irvine, Cancer research 2013, 1–12. [126] V. H. Engelhard, J. L. Strominger, M. Mescher, S. Burakoff, Proceedings of the National Academy of Sciences of the United States of America 1978, 75, 5688–91. [127] C. J. Turtle, S. R. Riddell, The Cancer Journal 2010, 16, 374–81. [128] M. Mescher, J. Curtsinger, in Vaccinology: Principles and Practice, 2012, pp. 286–299. [129] G. Gregoriadis, Trends in biotechnology 1995, 13, 527–37. [130] V. P. Torchilin, Nature reviews. Drug discovery 2005, 4, 145–60. [131] B. J. Prakken, R. Samodal, T. D. Le, F. Giannoni, G. P. Yung, J. Scavulli, D. Amox, S. Roord, I. de Kleer, D. Bonnin, et al., Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 4228–33. [132] R. Zappasodi, M. Di Nicola, C. Carlo-Stella, R. Mortarini, A. Molla, C. Vegetti, S. Albani, A. Anichini, A. M. Gianni, Haematologica 2008, 93, 1523–34. 47 [133] Q. Ding, J. Chen, X. Wei, W. Sun, J. Mai, Y. Yang, Y. Xu, Pharmaceutical research 2013, 30, 60– 9. [134] D. J. Irvine, Nature materials 2011, 10, 342–3. [135] T. Jin, P. Pennefather, P. I. Lee, FEBS letters 1996, 397, 70–4. [136] K. Shen, J. Tsai, P. Shi, L. C. Kam, Journal of the American Chemical Society 2009, 131, 13204– 5. [137] S. a Goldstein, M. F. Mescher, Journal of immunology (Baltimore, Md. : 1950) 1986, 137, 3383–92. [138] A. Z. Dudek, M. F. Mescher, I. Okazaki, V. T. Math, X. Luo, J. M. Curtsinger, J. S. Miller, American journal of clinical oncology 2008, 31, 173–81. [139] M. J. Deeths, M. F. Mescher, European journal of immunology 1997, 27, 598–608. [140] M. J. Deeths, M. F. Mescher, European journal of immunology 1999, 29, 45–53. [141] B. L. Musgrave, C. L. Watson, S. M. M. Haeryfar, C. a Barnes, D. W. Hoskin, Cellular immunology 2004, 229, 1–12. [142] K. Schilbach, G. Kerst, S. Walter, M. Eyrich, D. Wernet, R. Handgretinger, W. Xie, H.-G. Rammensee, I. Müller, H.-J. Bühring, et al., Blood 2005, 106, 144–9. [143] X. Jiang, X. Lu, R. Liu, F. Zhang, H. Zhao, Clinical cancer research : an official journal of the American Association for Cancer Research 2007, 13, 7329–34. [144] S. Caserta, P. Alessi, J. Guarnerio, V. Basso, A. Mondino, Cancer research 2008, 68, 3010–8. [145] X. Lu, X. Jiang, R. Liu, H. Zhao, Z. Liang, Cancer letters 2008, 271, 129–39. [146] D. Rudolf, T. Silberzahn, S. Walter, D. Maurer, J. Engelhard, D. Wernet, H.-J. Bühring, G. Jung, B. S. Kwon, H.-G. Rammensee, et al., Cancer immunology, immunotherapy : CII 2008, 57, 175– 83. [147] C. Shen, K. Cheng, S. Miao, W. Wang, Y. He, F. Meng, J. Zhang, Immunology letters 2013, 150, 1–11. [148] J. M. Curtsinger, M. F. Mescher, Current opinion in immunology 2010, 22, 333–40. [149] M. Oelke, J. P. Schneck, Immunologic research 2010, 47, 248–56. [150] B. L. Levine, W. B. Bernstein, M. Connors, N. Craighead, T. Lindsten, C. B. Thompson, C. H. June, Journal of immunology (Baltimore, Md. : 1950) 1997, 159, 5921–30. [151] L. G. Lum, a V LeFever, J. S. Treisman, N. K. Garlie, J. P. Hanson, Journal of immunotherapy (Hagerstown, Md. : 1997) 2001, 24, 408–19. 48 [152] A. K. Thomas, M. V. Maus, W. S. Shalaby, C. H. June, J. L. Riley, Clinical Immunology 2002, 105, 259–272. [153] F. Ito, A. Carr, H. Svensson, J. Yu, A. E. Chang, Q. Li, Journal of immunotherapy (Hagerstown, Md. : 1997) 2003, 26, 222–33. [154] M. V Maus, J. L. Riley, W. W. Kwok, G. T. Nepom, C. H. June, Clinical Immunology 2003, 106, 16–22. [155] M. Oelke, M. V Maus, D. Didiano, C. H. June, A. Mackensen, J. P. Schneck, Nature medicine 2003, 9, 619–24. [156] M. Oelke, J. P. Schneck, Clinical immunology (Orlando, Fla.) 2004, 110, 243–51. [157] H. Zhang, K. M. Snyder, M. M. Suhoski, M. V Maus, V. Kapoor, C. H. June, C. L. Mackall, Journal of immunology (Baltimore, Md. : 1950) 2007, 179, 4910–8. [158] C. Schütz, M. Fleck, A. Mackensen, A. Zoso, D. Halbritter, J. P. Schneck, M. Oelke, Blood 2008, 111, 3546–52. [159] S. Ugel, A. Zoso, C. De Santo, Y. Li, I. Marigo, P. Zanovello, E. Scarselli, B. Cipriani, M. Oelke, J. P. Schneck, et al., Cancer research 2009, 69, 9376–84. [160] M. Durai, C. Krueger, Z. Ye, L. Cheng, A. Mackensen, M. Oelke, J. P. Schneck, Cancer immunology, immunotherapy : CII 2009, 58, 209–20. [161] Z. M. Ndhlovu, M. Oelke, J. P. Schneck, D. E. Griffin, Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 3669–74. [162] S. a Fuertes Marraco, P. Baumgaertner, A. Legat, N. Rufer, D. E. Speiser, Journal of immunological methods 2012, 385, 90–5. [163] S. Demento, E. R. Steenblock, T. M. Fahmy, Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2009, 2009, 1161–6. [164] H. Han, J.-R. Peng, P.-C. Chen, L. Gong, S.-S. Qiao, W.-Z. Wang, Z.-Q. Cui, X. Yu, Y.-H. Wei, X.-S. Leng, Biochemical and biophysical research communications 2011, 411, 530–5. [165] T. R. Fadel, N. Li, S. Shah, M. Look, L. D. Pfefferle, G. L. Haller, S. Justesen, C. J. Wilson, T. M. Fahmy, Small (Weinheim an der Bergstrasse, Germany) 2012, 1–7. [166] S. Mandal, Z. H. Eksteen-Akeroyd, M. J. Jacobs, R. Hammink, M. Koepf, A. J. A. Lambeck, J. C. M. van Hest, C. J. Wilson, K. Blank, C. G. Figdor, et al., Unpublished n.d. [167] T. Kim, H. Lee, Y. Kim, J.-M. Nam, M. Lee, Chemical communications (Cambridge, England) 2013, 49, 3949–3951. [168] F. Giannoni, J. Barnett, K. Bi, The Journal of Immunology 2005, 174, 3204–3211. [169] K. Murphy, Janeway’s Immunobiology, 8th Edition, Garland Science, 2012. 49 [170] E. Koffeman, E. Keogh, M. Klein, B. Prakken, S. Albani, Methods in molecular medicine 2007, 136, 69–86. 50