EXPERIMENT 4 Spectroscopy
advertisement

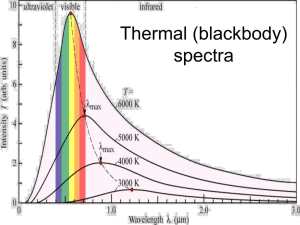
EXPERIMENT 4 Spectroscopy Atomic Emission and the Light from Stars Written by Dr. Steve Keller, University of Missouri – Columbia Chemistry Department “Circumstantial evidence can be overwhelming. We have never seen an atom, but we nonetheless know that it must exist” – I. Asimov. “Twinkle, Twinkle Little Star, how I wonder what you are” –Jane Taylor Introduction We have discussed in class the structure of atoms and the quantization of energy, which lead to the observation that, when excited, different elements give off a characteristic set of colors. The colors that we see are either those that are given off by a particular illuminating object (like a light bulb or star), or those that are reflected (i.e. not absorbed) by an object (like an apple or a shirt). As an example from biology, you may know that one of the molecules responsible for the green color of a plant is chlorophyll. The chlorophyll molecule efficiently absorbs blue and red light (the high and low energy regions of visible light) but does not absorb green and yellow light. What we see is the reflected (not absorbed) light is so the leaf appears green. Figure 0.1 Absorption Spectrum of Chlorophyll a. The “spectral signature” (the various techniques are generally described as spectroscopy) of a particular substance is used by scientists in a variety of ways, from analyzing the composition of an unknown alloy, to quantifying amount of chlorophyll in plants or iron in blood, to measuring the temperature of distant stars. The Pleiades cluster of stars is particularly well suited for this investigation in that the stars located there are all approximately the same age, so their spectra are primarily a function of their temperature. The major classifications of stars are shown in the Table. Specifically in the this lab you will look at the intensities of H and He lines in the spectra of two stars, and from comparison to the known spectral classes shown in the table above be able to decide to which category your stars belong. Note: The following parts need not be performed in any particular order. While you will need the calibration data fom Part I for Parts II and III, the corrections (if needed) can be done at any time. You can also do the telescope simulation at any time as well. Type Color Approximate Surface Temperature O Blue > 25,000 K B Blue A Main Characteristics Examples Singly ionized helium lines either in emission or absorption. Strong ultraviolet continuum. 10 Lacertra 11,000 – 25,000 Neutral helium lines in absorption. Rigel Spica Blue 7,500 – 11,000 Hydrogen lines at maximum strength for A0 stars, decreasing thereafter. Sirius Vega F Blue to White 6,000 – 7,500 Metallic lines become noticeable. Canopus Procyon G White to Yellow 5,000 – 6,000 Solar-type spectra. Absorption lines of neutral metallic atoms and ions (e.g. singly-ionized calcium) grow in strength. Sun Capella K Orange to Red 3,500 – 5,000 Metallic lines dominate. Weak blue continuum. Arcturus Aldebaran M Red < 3,500 Molecular bands of titanium oxide noticeable. Betelgeuse Antares Table 0.1 Classification of Stars in the Main Sequence. Part I: Use and Calibration of the Spectroscope Shown in Figure 0.2 is a schematic of the scopes we will be using. You will look through the slit in the short side of the scope while making sure the light is directly in front of the entrance slit on the opposite side. The slanted edge should be on the right side and the label on the scope should be on the top when you are observing spectra. First, point the ‘scope at the fluorescent lights in the room; you should see several colored lines near the scale bar on the right side as you view into the scope. If you see nothing, let your TA know. Figure 0.2 Use of your spectroscope. Point the ‘scope at the light source, but look through the narrow end at the scale printed on the inside. Observe the lines from the mercury vapor lamp. You should be able to see three or maybe four lines. The 405 nm line is very close to the detection limit of your eye, so the highest energy line that you see may be the blue 436 nm line. You should also see the green line at 546 nm and the yellow line at 578 nm. Caution! The mercury lamp emits ultraviolet radiation that may be damaging to your eyes. Only look into the mercury lamp while wearing your lab goggles. Record the colors and wavelengths of the lines you observe from the lamp. If the wavelengths you record from your spectroscope are significantly off from these values, inform your TA and you can replace your spectroscope. Sometimes the diffraction grating or the scale slips. If they are pretty close (but not exact), construct a graph of observed wavelengths versus the reference wavelengths given above. Calculate the best-fit straight line through the data (Excel or the Graphical Analysis program on the lab computers will do this). This will give you a calibration equation with which you can correct the wavelengths that you measure for H and He. Part II: Emission Spectra Observe the hydrogen and helium lamps with the spectroscope. For both elements, record the color and wavelength as precisely as you can. Both members of each group should observe both lamps. Using your calibration graph (if necessary) calculate the correct positions of the H and He lines that you see. Part III: Absorption Spectra There are several colored solutions in clear glass bottles. You will need to observe the spectra generated by these solutions when illuminated from behind by a white, incandescent light. You may want to view the white light by itself first to get a good comparison. These solutions will not give the sharp lines of the emission but rather broader regions where the light is absorbed. Record the ranges of wavelengths that you see in the spectrum of the solutions. As part of your observations, comment on the relationship between the color of the solutions that you see to the colors of light present in the absorption spectrum. Part IV: Interpretation of Stellar Spectra In this simulation, you will collect emission data from a star and assign it to a category based on its temperature. We are going to be looking at the star cluster known as the Pleiades or Seven Sisters Start the “Stellar Spectra” Program from the desktop icon. Select “File” from the pull-down menu and “Log-In”. Enter the names of the persons in your group (you can leave the table # blank). Once you have finished logging in, go back to the File menu and select “Run” and then “Take Spectra”. The first thing you must do is open the dome. Collecting Data. You should now have “virtual” control of the telescope. You will notice that the star field will move at regular intervals (due to the spinning of the Earth). To render the stars stationary, turn on “Tracking”. We will be investigating stars in the Pleiades Cluster this week. Go to the top “Field…” pull down menu and Select “Pleiades”. Figure 0.3 The Pleiades star cluster, also known as the Seven Sisters, is more than 300 light years from Earth. Nature 427, 299-300 (22 January 2004) With the directional buttons you can move the red box to an area containing one or more stars of your choosing. To align the spectrometer, select “Change View” and a narrow red slit will be shown in the middle of the screen. Move the object star within the slit again with the directional arrows. When the star is centered, press “Take Reading”, which will call up a graph of intensity versus wavelength ( Note: 10 Å = 1 nm). To begin data collection, select “Start/Resume Count” in the upper left-hand corner. You will see peaks corresponding to the wavelengths most emitted by the star. When you are happy with the data, select “Save”, and enter the last three digits of the object ID number (e.g. HD23408 becomes 408 so that you can retrieve it later). Print your spectrum. Return to the Telescope View . Spectral Analysis. Many standard spectra of star types have already been collected. Your task is to identify the class and therefore the temperature of the stars you chose. From the telescope view, go to “File: and select “Run…Classify Spectra”. That will bring up three blank spectra stacked on one another. You can then load one of your saved spectra, and a set of standard spectra with which you can make comparisons. First, under “File”, select “Atlas of Standard Spectra”, and from the list shown, select “Main Sequence”. This will bring up another window with 13 different spectra. The hottest stars are at the top, and the coolest (temperature) are at the bottom. To display your data, select “Unknown Spectra” from the “File” menu, and then “Saved Spectra”. Double-click on one of your spectra and it will appear in the middle box. Use the cursor to determine the wavelengths corresponding to the three most intense lines you see. Then pan through the spectra of main sequence stars to find one that matches yours the best. When you have a good match, go to “Classification Results” and select “Record” and filling in the information requested. Then you can print out everything for later reference. Find another star (with a different spectrum) and do the same collection and analysis.