Group Theory: Relevancy to Chemistry
advertisement

Group Theory
Relevancy to Chemistry
4/15/2009
MTH 4110
Joel Guttormson
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Introduction
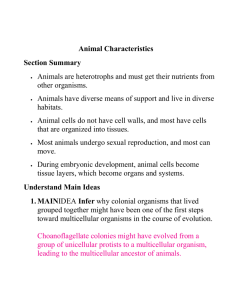
Group theory is more applicable than one may realize. In fact, it is integral in the
understanding of chemistry. Specifically, this paper will focus on the group theory of chemical
bonds. Group theory greatly reduces the level of mathematical difficulty and furthers an
understanding of the complex interactions between atomic orbitals that form molecular bonds.
This paper will contain a brief discussion on the construction of character tables, which are
directly related to group operation tables to enable a more coherent explanation of the relevancy
of group theory to chemical bonds. The objective of this paper will be to show the deep
interconnectedness of group theory and chemical bonding in a way that mathematicians as well
as chemists can understand it.
Symmetry and Point Groups
Though most mathematicians know what symmetry is, it is useful to see how chemists
define symmetry. Symmetry is, “that property of a body (or pattern) by which the body (or
pattern) can be brought from an initial spatial position to another, indistinguishable position by
means of a certain operation, known as a symmetry operation.” (Ladd, 1998, p. 5) Though this
definition is worded quite differently from our mathematical notions of symmetry, it is
nonetheless equivalent. Another useful definition is that of something called a point group. A
point group, the use of the word group is justified as will be shown later, is “a set of symmetry
operations, the action of which leaves at least one point of the body invariant, or unmoved”
(Ladd, 1998, p. 46). In this discussion, it will be useful to focus our examination on a simple and
1|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
well-known molecule, water. It is common knowledge that water is comprised of two hydrogen
atoms and one oxygen atom, forming a molecule. At first, you may be thinking, as I did, that the
water molecule follows the same symmetry pattern as that of the group 𝒮3 or of the symmetry
properties of an equilateral triangle. However, the symmetry of the water molecule is more
complex than that due to something called an orbital. An orbital is “a specific region of
an atom” that “can contain two electrons with paired spins.” (Anne Marie Helmenstine,
2009)Orbitals, in diagrams, show the charge associated with the atom in the molecule (see Figure
1 below, (Kettle, 2007, p. 20)). These orbitals can have a significant effect on the group
structure. Whereas an equilateral triangle can be “flipped” 180° about a vertical axis, call it z,
and that symmetry operation would be considered an identity operation, this is not the case with
the water molecule. Not only do orbitals complicate the matter of symmetry, rather it is further
complicated by the undeniable fact that, while an equilateral triangle is a two-dimensional object,
a molecule is, at least, a three-dimensional object. However, due to this complication of three
dimensions we will not consider auxiliary axes such as the lines 𝑦 = 𝑥 𝑜𝑟 𝑧 = 𝑦. Thus, we will
only considered what can be called the “normal” axes of x, y and z with their standard positions
and directions intact.
Figure 1
2|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
The Water Molecule: A Symmetry Group
We know water is comprised of three atoms; two hydrogen and one oxygen. The
arrangement, (see Figure 2 below), allows us to visually examine the symmetry group properties
of the water molecule.
Figure 2
As stated previously, the identity element, E, is not the 180° rotation about the z-axis, but a 360°
rotation about the z-axis. There are of course, other symmetry operations. 𝐶2 is the operation
that rotates the water molecule 180° about the z-axis. 𝜎𝑣 is an operation that requires a more
sophisticated definition. “The 𝜎𝑣 operation…leaves the phases unchanged (although the ‘front’
and ‘back’ of each lobe are interchanged.” (Kettle, 2007, p. 20) It has the effect of putting a
“mirror plane” through the molecule, hence the subscript v for vertical. For further visual
reference, it “has the effect of interchanging the two hydrogen atoms.” (Kettle, 2007, p. 15) The
𝜎′𝑣 operation, like the 𝐶2 operation, reverses the phases but does so by reversing the lobe of just
the oxygen atom (see Figure 3 below).
3|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Figure 3
Now that we have these operations, we can develop a multiplication table to see how the
different operations interact with each other.
By visual inspection of Table 1 below, it is clear that it represents a group, by the
informal definition. That is, all elements are represented in the table, no element appears twice
in the same row or column, and there is an identity element. However, this is not sufficient and
thus we must formally prove that this set, call it 𝒯 = {𝐸, 𝐶2 , 𝜎𝑣 , 𝜎′𝑣 } is a group. For 𝒯 to be a
group it must be nonempty with a single binary operation and “satisfy the following axioms: 1.
Closure: 𝐼𝑓 𝑎 ∈ 𝐺 𝑎𝑛𝑑 𝑏 ∈ 𝐺, 𝑡ℎ𝑒𝑛 𝑎 ∗ 𝑏 ∈ 𝐺.; 2. Associativity: 𝑎 ∗ (𝑏 ∗ 𝑐) = (𝑎 ∗ 𝑏) ∗
4|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
𝑐 ∀ 𝑎, 𝑏, 𝑐 ∈ 𝐺.; 3. There is an element 𝑒 ∈ 𝐺 𝑠𝑢𝑐ℎ 𝑡ℎ𝑎𝑡 𝑎 ∗ 𝑒 = 𝑎 = 𝑒 ∗ 𝑎 ∀𝑎 ∈ 𝐺.; 4. For each
𝑎 ∈ 𝐺 there is an element 𝑑 ∈ 𝐺 such that 𝑎 ∗ 𝑑 = 𝑒 𝑎𝑛𝑑 𝑑 ∗ 𝑎 = 𝑒.” (Hungerford, 1997, p. 163)
Table 1
𝐶2𝑣
E
𝑪𝟐
𝝈𝒗
𝝈′𝒗
E
E
𝐶2
𝜎𝑣
𝜎′𝑣
𝑪𝟐
𝐶2
E
𝜎′𝑣
𝜎𝑣
𝝈𝒗
𝜎𝑣
𝜎′𝑣
E
𝐶2
𝝈′𝒗
𝜎′𝑣
𝜎𝑣
𝐶2
E
𝑃𝑟𝑜𝑝𝑜𝑠𝑖𝑡𝑖𝑜𝑛 1: 𝒯 𝑖𝑠 𝑎 𝑔𝑟𝑜𝑢𝑝.
Proof: Define 𝒯 = {𝐸, 𝐶2 , 𝜎𝑣 , 𝜎′𝑣 }. Then 𝒯 is nonempty. The binary operation of 𝒯 is
“symmetry multiplication”, that is, the consecutive application of symmetry operations. 𝒯 is
closed by observing that in multiplication table ∄an element in the table that is not in 𝒯.
Symmetric operations are known to be associative. By observing the table, each element is its
own inverse, that is, 𝐸 ∙ 𝐸 = 𝐸, 𝐶2 ∙ 𝐶2 = 𝐸, 𝜎𝑣 ∙ 𝜎𝑣 = 𝐸, 𝜎′𝑣 ∙ 𝜎′𝑣 = 𝐸. There is an identity
element, 𝐸 ∈ 𝒯. Again by observing the table, the first leftmost column and the first row show
that 𝐸 is the identity since all elements times 𝐸 are equal to themselves. Thus, 𝒯 is a group.∎
From now on, we will denote the group 𝒯 as 𝐶2𝑣 . We now have a fascinating result at hand.
The water molecule, because of its symmetry, forms a group1. Not only is it a group, but it is
also a point group, by definition, since for any rotation we leave the oxygen atom fixed. Note
Interestingly, 𝐶2𝑣 is isomorphic to 𝑈8 = {1,3,5,7} (the set of units in ℤ8 ). Since this result has no bearing on the
remainder of the material, the proof of this result is left to the reader.
1
5|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
that the interchange of the lobes of the oxygen atom does not negate or contradict the assertion
that 𝐶2𝑣 is a point group or that it fits the definition of such. Now it is useful to expand upon the
aforementioned in the direction of the chemistry in order to show more correlation between it
and the mathematics.
Character Tables
Mathematicians would find the above satisfactory in the description of the superficial group
structure of 𝐶2𝑣 mentioned and developed above. However, chemists require more. Recall the
elements of 𝐶2𝑣 = {𝐸, 𝐶2 , 𝜎𝑣 , 𝜎′𝑣 }. In our development, it was left out that these operations have
what are called multipliers associated with them. These multipliers deal with whether or not the
phases are changed. For 𝐸 𝑎𝑛𝑑 𝜎𝑣 , it can be easily observed that the phase does not change
under those operations. These operations are called “symmetric”. An operation that is
“symmetric with respect to rotation by 2𝜋⁄𝑛” is given the multiplier value of 1. Those that are
not symmetric in this way, i.e. the phases change due to application of the symmetry operation,
are called “antisymmetric” and are given the multiplier value of -1. (Cotton, 1990, pp. 90-91)
These notions of “symmetric” and “antisymmetric” require us to shift our perspective and also
consider more advanced view of the phase patterns of the water molecule because as defined
they are not complete and do not really tell the whole story. Figure 4 below2 shows the
orientation we will be considering. It is good also to note that we are talking merely about the
phases of the oxygen atom in the molecule. The additional complexity of the consideration of
the hydrogen atoms of the molecule is outside the scope of this paper.
2
Figure 4 shows a shift in orientation with the main axis being the x-axis rather than the z-axis.
6|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Figure 4
At first glance, one will notice what appear to be two “extra” phases or lobes. For simplicity
only realize that our initial view left out that inside the oxygen molecule there are protons (and
neutrons of course, but they do not affect the charge of the atom) that may be configured in
different ways as to produce several configurations of the oxygen atom’s phases. The
configuration, shown above in Figure 4, is used for ease of understanding though it is
acknowledged that other configurations do exist. Below, in Figure 5, we see the symmetry
operations at work, however this time we get a clearer picture of what is happening. In E, we see
that not much has changed from our original look at it. The lobes do not change. However, in
𝜎𝑣 𝑎𝑛𝑑 𝜎′𝑣 we see that the two “smaller” lobes (phases) do in fact change. Thus, the above
description of what is going, as said, is not complete or for that matter, entirely correct because
we had, essentially, an incomplete picture. Then, our delegation of the numbers 1 and -1 to
certain elements of 𝐶2𝑣 (i.e. symmetry operations) has, using the definitions of “symmetric” and
“antisymmetric”, E and 𝐶2 as symmetric operations and 𝜎𝑣 , 𝜎′𝑣 as antisymmetric operations.
Why is this so? The answer lies within examination of Figure 5 below.
7|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Figure 5
If we examine the new, “smaller” lobes, we can see that in 𝜎𝑣 𝑎𝑛𝑑 𝜎′𝑣 the charge of the lobes is
opposite that of the molecule before the operation. Whereas, in E and 𝐶2 the lobes not only
remain unchanged but are virtually, but not identically, equal to each other. Now, we can begin
translating Table 1 into what is called a “character table”. A character table is a simple way to
tabulate the four representations of the ways that functions may transform in 𝐶2𝑣 . (Ladd, 1998, p.
87) However, before we can create a full character table for 𝐶2𝑣 we must first create the most
important piece of the character table, which is its “irreducible representations”. It is in this
piece that the 1’s and -1’s become useful. Kettle offers the best explaination of how to translate
8|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
this notion and incorperate it with our knowledge of Table 1. “Everywhere in [Table 1] that the
operation E is listed, replave it by the number with which it is associated”. (Kettle, 2007, p. 40)
Table 2
E
1
𝑪𝟐
𝝈𝒗
𝝈′𝒗
1
1
1
1
1
-1
-1
1
-1
1
-1
1
-1
-1
1
Then, taking analogous information from above, we can complete the table as show above in
Table 2. With further substitution and the addition of {𝐴1 , 𝐴2 , 𝐵1 , 𝐵2 } which are the irreducible
representations of the individual lobes. (Ladd, 1998, p. 85) In other words, each row is an
irreducible representation of an operation and we represent each as above with the A’s and B’s.
Now it is possible to create our character table, Table 3 (Kettle, 2007, p. 42) from the above,
accumulated information.
Table 3
𝐶2𝑣
E
𝑪𝟐
𝝈𝒗
𝝈′𝒗
𝐴1
1
1
1
1
𝐴2
1
1
-1
-1
𝐵1
1
-1
1
-1
𝐵2
1
-1
-1
1
9|Page
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Conclusion
Although the above examination was brief and touches only on a limited range of topics and uses
of group theory in chemistry, it shows that group theory is an integral part of the understanding
and application of chemistry, especially when it comes to understanding the fundamentals of
bonding and interactions between atoms.
Furthermore, the above has shown that one particular aspect of chemistry, namely
character tables, stand alone as one of the most important fundamentals to learn when studying
chemistry and are completely dependent on concepts in group theory. This finding shows that
even the most abstract of algebra can be applicable. Probably the most interesting, though not
entirely practical, result from the above is that the water molecule, with symmetry operations
actually forms a group and that this group is isomorphic to an abstract, seemingly purely
mathematical group, 𝑈8 (the units of the group ℤ8 ). Another remarkable result or at least a
consequence is, that the physical properties that chemistry grapples with can severely influence
group structure and behavior. As with the example of the water molecule, the complexity added
by charge and the positions of the phases (lobes), even just those of the oxygen atom, both
greatly focus and expand our purely mathematical notions of groups and group structure. I find
it astonishing that the physical world in which we live can be defined both by the most elegant
equation or formula and by the most abstract and theoretical of concepts. I can think of no better
example of this than the application of group theory to chemistry because chemistry, more than
most science, really is part of our everyday lives. This, in the end, makes group theory part of
our everyday lives as well.
10 | P a g e
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Figures and Tables
Table 1 (Kettle, 2007, p. 39) ...........................................................................................................................................5
Table 2 (Kettle, 2007, p. 40) ...........................................................................................................................................9
Table 3 (Kettle, 2007, p. 42) ..........................................................................................................................................9
Figure 1 (Kettle, 2007, p. 20) .........................................................................................................................................2
Figure 2 (Kettle, 2007, p. 14) .........................................................................................................................................3
Figure 3 (Kettle, 2007, p. 21) .........................................................................................................................................4
Figure 4 (Kettle, 2007, p. 30) .........................................................................................................................................7
Figure 5 (Kettle, 2007, p. 31) .........................................................................................................................................8
Bibliography
Anne Marie Helmenstine, P. (2009). Orbital Definition: Chemistry Glossary Definition of
Orbital. Retrieved 4 18, 2009, from About.com:
http://chemistry.about.com/od/chemistryglossary/a/orbitaldef.htm
Cotton, F. A. (1990). Chemical Applications of Group Theory. New York: John Wiley & Sons,
Inc.
Hungerford, T. W. (1997). Abstract Algebra: An Introduction. Thomson Learning, Inc.
Kettle, S. F. (2007). Symmetry and Structure: Readable Group Theory for Chemists. Chichester:
John Wiley & Sons Inc.
Ladd, M. (1998). Symmetry and Group Theory in Chemistry. Chichester: Horwood Publishing.
11 | P a g e
Joel Guttormson
Term Paper
MTH 4110
4/15/2009
Table of Contents
Introduction ..................................................................................................................................... 1
Symmetry and Point Groups ........................................................................................................... 1
The Water Molecule: A Symmetry Group...................................................................................... 3
Character Tables ............................................................................................................................. 6
Conclusion .................................................................................................................................... 10
Figures and Tables ........................................................................................................................ 11
Bibliography ................................................................................................................................. 11
12 | P a g e