Difference in refractive index between tap water and salt water
advertisement

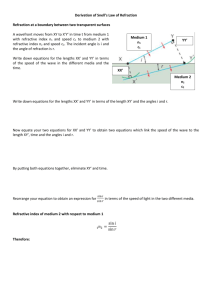
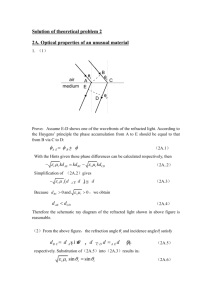
Difference in refractive index between tap water and salt water L. Snel & N. Chekrouni Het 4e Gymnasium, Amsterdam Summary When light travels from air to a liquid the direction of light changes. This phenomenon is called refraction. Each medium has its own refractive index. The purpose of the experiment was to find out if the refractive index of salt water differs to the refractive index of tap water. Four different solution had been taken for the experiment. After measuring the refractive indices, the refractive index of salt water turned out to be bigger than the refractive index of tap water. Introduction Refraction is the bending of a (light)wave when it passes from a fast medium to a slower one. The amount of bending depends on the index of refraction, which differs for each sort of medium. We know that there is a constant ratio between the sine of the angle of refraction θ1 and the sine of the angle of refraction θ2 when a light beams from vacuum and enters a transparent medium, according to Snell's Law. This constant ratio is also called the refractive index n: This raises the question: Does tap water differs in refractive index from salt water? To make salt water, sodium chloride has been used. Sodium chloride dissolves easily in water and separates this into Na+ and Cl- ions according to the following equation: NaCl (aq) Na+ (aq) + Cl- (aq) Refraction When the angle of refraction θ2 is bigger than 90°, total reflection occurs. Because an angle bigger than 90 NaCl is separated into Na+ and Cldegrees would prevent us to determine the angle of refraction θ2, there is a limit g to angle of refraction θ1. Research showed that the maximum amount of This critical angle is determined by salt that dissolves in one liter of tap water is 359 grams. In order to make a solution in which the maximum amount of NaCl can dissolve you only need 80% of that amount. where n1 and n2 are the respective refractive indices. Experimental procedure and data Each and every medium has its own refractive index. When light travels from air to water, the water will analysis refract differently then when light refracts from air to Four identical measuring cylinders had been taken. The first measuring cylinder was filled with 1 liter another substance. of tap water. The second measuring cylinder was also filled with 1 liter of tap water and 287,2 grams of salt was added, which is 80% of the maximum amount of salt that can dissolve in water. The third measuring cylinder was filled with 0,5 liter of the second solution, and then added 0,5 liter of tap water, so that the third solution was two times more diluted than the second solution. In the fourth measuring cylinder 0,5 liter of our third solution had been added and then we added 0,5 liter of tap water again, so that the fourth solution was a two times more diluted than our third solution. The supplies that were used for this experiment were: - A bowl with a magnetic strip - A magnetic protractor - A laser light attached to a magnet The protractor was attached to a magnetic board and the bowl was filled (also attached to the board) with the first solution, with such precision that the solution surface was exactly on the x-axis of the protractor. Then the laser light also was attached to the magnetic board on the side of the negative x-axis and a positive y-axis. The angle of refraction θ1 was variable but in such manner that no reflection occurred (smaller than the critical angle of 48,6°). Then the angle in the solution was been observed, by looking at it perpendicularly. In this way angle of refraction θ2 was observed. These observations were repeated several times so that the results would be as accurate as possible. After finishing the observations with the first liquid (tap water) the experiment continued the same way as written above but with a difference in liquid/solution. Results Each experiment for each solution has be repeated three times, each time with a different angle of refraction n1. Water/sol.1 Sol.2 Sol.3 Sol.4 st 1 Sin 30°/ sin Sin 30°/ Sin Sin 30°/ 22° = 1,33 sin 20°= 30°/sin sin 23° 1,46 22 °= = 1,28 1,33 2nd Sin 35° / sin 25° = 1,35 Sin 35°/ sin 25° = 1,36 Sin 35° / sin 26° = 1,31 Sin 35°/ sin 26° = 1,31 3rd Sin 40°/ sin 29° = 1,33 Sin 40°/ sin 29 °= 1,32 Sin 40° / sin 30° = 1,29 Sin 40°/ sin 30° = 1,29 avera ge 1,337 1,381 1,309 1,291 Table 1: refractive indices of four solutions Table 1 shows the results of the experiments. The experiment is repeated three times so at the end the average of the three experiments could be taken. This gives better and more accurate results. Keep in mind that solution 1 tap water is, and that solution two times stronger is than solution 3 and that solution 4 contains the least NaCl. Conclusion / discussion The conclusion that can be drawn from the results is that salt water definitely has a higher refractive index then tap water. This occurs because of the separation of NaCl into Na+ and Cl- ions. Those ions are diffused all over in the water, therefore it becomes more difficult for the water to bend the incoming light beam like it does when it is only tap water. Also it is noteworthy that the difference in refractive index between the less dissolved solutions is lower than expected. This is due to the low amount of NaCl that is dissolved in water and doesn’t show such a powerful effect as the ones with a higher concentration of salt. It is strange that the third and fourth solution have a lower refractive index than water. Perhaps other salts could have been used, in order to achieve some more interesting results, with perhaps also differences in refractive index. Also the accuracy of the experiment could have been more precise, as noticeable from the abnormalities in the results. This is due to the difficulty of keeping the bowl straight on x-axis of the protractor when it is filled with water or another solutions and becomes more heavy. Bibliography 1. BINAS table 18B 2. http://nl.wikipedia.org/wiki/Brekingsind ex 3. http://en.wikipedia.org/wiki/Refractive_i ndex 4. Iru, P., Luib, A., & Nelem, D. (2010) Journal of anorgano chemistry. The effect of NaCl (s) on ice