Standard Formation enthalpies of methyladamantanes Apendina А
advertisement
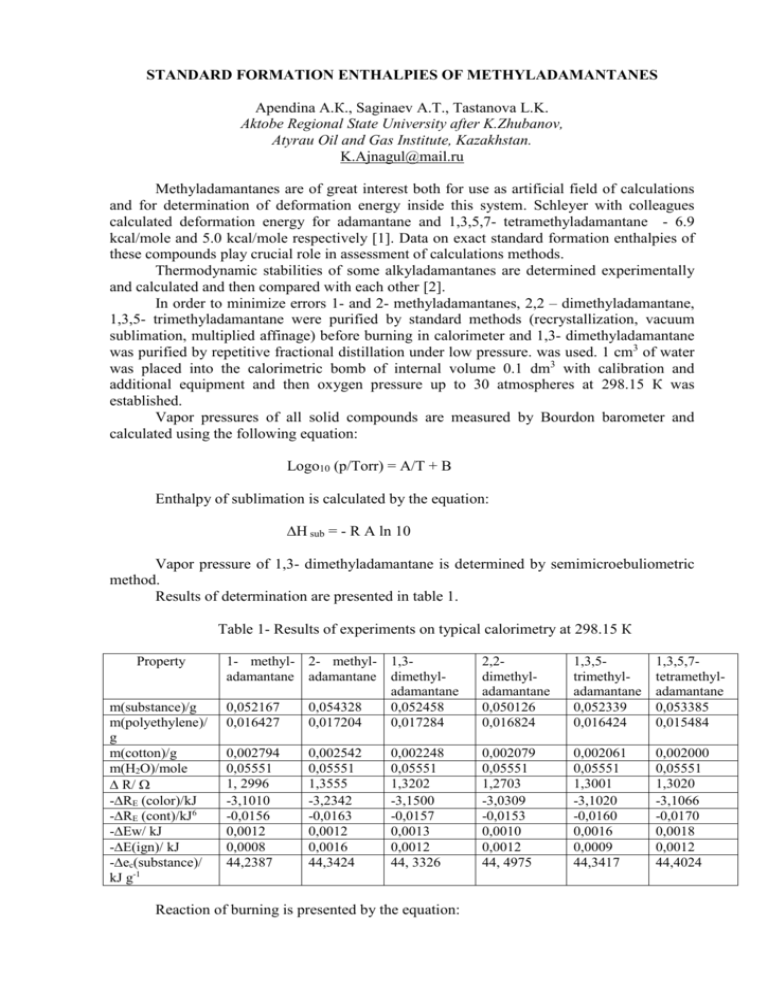
STANDARD FORMATION ENTHALPIES OF METHYLADAMANTANES Apendina А.К., Saginaev А.Т., Tastanova L.K. Aktobe Regional State University after K.Zhubanov, Atyrau Oil and Gas Institute, Kazakhstan. K.Ajnagul@mail.ru Methyladamantanes are of great interest both for use as artificial field of calculations and for determination of deformation energy inside this system. Schleyer with colleagues calculated deformation energy for adamantane and 1,3,5,7- tetramethyladamantane - 6.9 kcal/mole and 5.0 kcal/mole respectively [1]. Data on exact standard formation enthalpies of these compounds play crucial role in assessment of calculations methods. Thermodynamic stabilities of some alkyladamantanes are determined experimentally and calculated and then compared with each other [2]. In order to minimize errors 1- and 2- methyladamantanes, 2,2 – dimethyladamantane, 1,3,5- trimethyladamantane were purified by standard methods (recrystallization, vacuum sublimation, multiplied affinage) before burning in calorimeter and 1,3- dimethyladamantane was purified by repetitive fractional distillation under low pressure. was used. 1 cm3 of water was placed into the calorimetric bomb of internal volume 0.1 dm3 with calibration and additional equipment and then oxygen pressure up to 30 atmospheres at 298.15 К was established. Vapor pressures of all solid compounds are measured by Bourdon barometer and calculated using the following equation: Logo10 (p/Torr) = A/T + B Enthalpy of sublimation is calculated by the equation: ∆H sub = - R A ln 10 Vapor pressure of 1,3- dimethyladamantane is determined by semimicroebuliometric method. Results of determination are presented in table 1. Table 1- Results of experiments on typical calorimetry at 298.15 К Property m(substance)/g m(polyethylene)/ g m(cotton)/g m(H2O)/mole ∆ R/ -∆RE (color)/kJ -∆RE (cont)/kJ6 -∆Ew/ kJ -∆E(ign)/ kJ -∆ес(substance)/ kJ g-1 1- methyl- 2- methyl- 1,3adamantane adamantane dimethyladamantane 0,052167 0,054328 0,052458 0,016427 0,017204 0,017284 2,2dimethyladamantane 0,050126 0,016824 1,3,5trimethyladamantane 0,052339 0,016424 1,3,5,7tetramethyladamantane 0,053385 0,015484 0,002794 0,05551 1, 2996 -3,1010 -0,0156 0,0012 0,0008 44,2387 0,002079 0,05551 1,2703 -3,0309 -0,0153 0,0010 0,0012 44, 4975 0,002061 0,05551 1,3001 -3,1020 -0,0160 0,0016 0,0009 44,3417 0,002000 0,05551 1,3020 -3,1066 -0,0170 0,0018 0,0012 44,4024 0,002542 0,05551 1,3555 -3,2342 -0,0163 0,0012 0,0016 44,3424 0,002248 0,05551 1,3202 -3,1500 -0,0157 0,0013 0,0012 44, 3326 Reaction of burning is presented by the equation: CaHb + (a+b/4)O2 = aCO2 +1/2 b H2O Derivatives from standard molar energy of burning ∆Eb˚, standard molar enthalpy of burning ∆Нb˚ and standard molar enthalpy of formation ∆Нf˚ of compounds are presented in table 2. Table 2- Oscillating molar energies of condense state at 298.15 К Compounds 1- Methyladamantane 2- Methyladamantane 2,2- Dimethyladamantane 1,3- Dimethyladamantane 1,3,5- Trimethyladamantane 1,3,5,7- Tetramethyladamantane -∆Eb˚, kJ/mole -∆Нb˚, kJ/mole -∆Нf˚, kJ/mole 6647,1±0,4 6665,1±0,4 7311,5±0,6 7281,4±0,6 7913,6±1,0 8545,4±0,9 6658,4±0,4 6676,4±0,4 7324,1±0,6 7293,9±0,6 7927,4±1,0 8560,5±0,9 242,7±0,4 224,7±0,4 256,5±0,6 286,6±0,6 332,6±1,0 378,6±0,9 Derivatives from sublimation enthalpies of methyladamantanes are presented in table 3. Values of Тm are taken from before studied middle temperatures. Standard sublimation enthalpies, ∆Hs˚ (298.15К), are determined from the equation: ∆Hs˚(298.15К) = ∆Hs˚(Тm ) +(298.15 К – Тm ) (Сp˚(gas)- Сp˚(solid)) Table 3 – Sublimation enthalpies of methyladamantanes Compounds ∆Hs˚(Тm)/ kJ/mole Т/К 1- Methyladamantane 2- Methyladamantane 2,2- Dimethyladamantane 1,3- Dimethyladamantane 1,3,5- Trimethyladamantane 1,3,5,7- Tetramethyladamantane 16,0±0,2 16,1±0,2 17,2±0,2 16,2±0,2 18,2±0,2 19,5±0,2 300-340 300-340 300-360 -----300-360 310-350 ∆Hs ˚(298.15К)/ kJ/mole 16,2±0,3 16,3±0,3 17,6±0,3 16,2±0,3 18,6±0,3 20,0±0,3 Substitution of methyl groups in tertiary positions of adamantanes nuclei increases thermochemical stability. Literature 1. Engler E.M., Andose J.D., Schleyer P.v.R.// J.Am. Chem. Soc. 1993, 125, 8005 2. Сагинаев А.Т., Апендина А.К. // Вестник Атырауского Института Нефти и Газа , 2006, № 10, с. 125-129