Assignment #7
advertisement

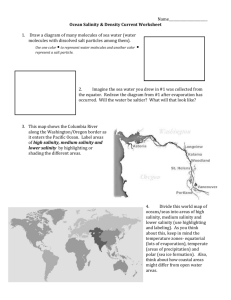
Assignment #7 & Study Guide Ocean Physics 1. The map in Figure 1 from your Seawater Temperature reading shows the distribution of surface temperatures in August in the major ocean basins. Refer to it for your answers to the following questions. a. Where is water of the greatest density formed? Explain your answer. b. Explain the “bending” of the isotherms in the North Atlantic Ocean. c. How would a depth-temperature curve from the Arctic regions differ from one at mid-latitudes or one from equatorial regions? 2. The table below lists temperature and salinity data for an oceanographic station off Point Conception, Southern California. Depth (meters) Temperature (°C) Salinity (‰) Depth (meters) Temperature (°C) Salinity (‰) 0 10 20 14.56 14.50 14.48 31.22 31.40 31.56 250 300 400 6.57 6.15 5.49 33.98 34.01 34.07 30 12.72 31.88 500 4.01 34.14 50 75 100 10.86 9.20 8.82 32.40 33.24 33.60 600 700 800 4.65 4.36 4.10 34.20 34.26 34.31 150 7.77 33.88 1000 3.51 34.41 200 7.10 33.94 a. Using the graph provided, plot the temperature data against depth. b. On your temperature-depth plot above, label the seasonal thermocline and the permanent thermocline. c. If this profile were taken during the winter, what changes in temperature might you expect to see in the uppermost 200 meters? 3. The figure below contains oceanographic data for an east-west trending line in the western North Atlantic Ocean off North Carolina. a. On the graph provided on the next page, plot the temperature versus depth data from the upper 1000 meters for the following three stations: NH 25 (100 km from shore), NH 65 (300 km from shore) and NH 105 (600 km from shore). Be sure to label each profile. b. Draw the 3°C, 4°C, 5°C, 6°C, 8°C , 10°C, 12°C , 15°C, 16°C , 18°C , 20°C, and 25°C contours (isotherms) for these temperature data on the diagram below. c. Between what distances from shore are the highest surface temperatures found? d. What isotherm surrounds those high surface temperatures? e. What do you notice about the water temperatures at depths below 2000 meters at each station? 4. Using the hydrostatic equation (P = gh), determine the pressure at 1400 meters depth. The average density of seawater is 1030 kg/m3 and the acceleration of gravity is a constant at 9.8 m/sec2. 5. If it takes 80 calories to convert 1 gram of ice to water, how much heat energy is required to convert a 3 kilogram block of ice to water? How much energy is required to return the water to ice? 6. Properties of a substance, such as density or specific heat, that do not involve chemical transformations, are called a. specific properties b. physical properties c. thermal properties d. atomic properties 7. The wind blowing over the ocean is an example of the transfer of a. potential energy b. thermal energy c. kinetic energy d. radiant energy 8. The transfer of heat between the ocean surface and the overlying atmosphere is an example of the exchange of a. heat or thermal energy b. potential energy c. chemical energy d. kinetic energy 9. Energy from the sun, or solar energy, is also known as a. thermal energy b. potential energy c. chemical energy d. radiant energy 10. Which temperature scale sets zero (0) as the freezing point of water? a. Kelvin b. Celsius c. Fahrenheit d. Reaumer 11. Temperature may be defined as a. the amount of heat contained my an object or system b. the capacity of an object or system to store heat c. a measure of the average kinetic energy of the molecules of an object or system d. all of the above 12. Heat exists only when a. two objects or systems are in thermal equilibrium b. objects or systems are above freezing c. two objects or systems have different temperatures d. an object is a liquid or a solid 13. Which weighs more, 500 molecules of water in a gaseous state or 500 molecules in a liquid state? a. 500 molecules in a gaseous state b. 500 molecules in a liquid state c. both weigh the same d. there is not enough information given to answer the question 14. The heat required to change the state of water from a solid to a liquid at 0 degrees C is called a. sensible heat b. latent heat c. internal heat d. exogeneous heat 15. The observation that beach sand at depth remains cool despite a very hot surface supports the conclusion that a. sand is a great conductor of heat b. sand is a poor conductor of heat c. heat convects poorly in sand d. heat convects well in sand 16. A diver at 99 feet will experience a. one atmosphere of pressure b. two atmospheres of pressure c. three atmospheres of pressure d. four atmospheres of pressure 17. A water sample taken from 1000 meters in the ocean will have a temperature at the surface (assuming no exchange with its surroundings) that is ______ than its temperature at 1000 meters. a. Cooler b. Warmer 18. Increases in temperature cause the density of seawater to _______ while increases in salinity cause seawater density to __________. a. decrease...decrease b. increase...increase c. decrease...increase d. increase...decrease 19. At present, the Earth-Sun distance is shorter in winter than summer. a. True b. False 20. The reason for the seasons? a. Earth's axial tilt b. the Earth-Sun distance c. the percentage of water in the Southern versus Northern hemisphere d. all of the above 21. The formation of a seasonal thermocline in temperate and polar waters is called a. Stratification b. Destratification c. Shoaling d. Salt fingering 22. Who are Daniel Fahrenheit, Antoine Ferchault de Reaumur, Anders Celsius, and Lord Kelvin? What are their respective contributions to thermometry? 23. List the three equations that allow conversion between Kelvin and Celsius, between Celsius and Fahrenheit, and between Fahrenheit and Celsius. 24. Have scientists ever reached Absolute Zero? How close have they come? 25. What is the difference between a protected reversing thermometer and an unprotected one? 26. What parameters are the robotic floats in the Argo system measuring? 27. Define Adiabatic Changes in temperature. Define Potential Temperature. 28. If substances pack their molecules closer together as they go from a gas to a liquid to a solid, why does solid water (ice) float in liquid water? 29. What is the density (in g/cm3) of pure water at 20°C? What is its density at 3.98°C? What is its density at 0°C? And what is its density when it has completed its phase change to ice at 0°C? (Use figure 5.4 from slide 17 of the lecture.) 30. Why does the ocean’s water usually appear blue-green? Sometimes the color of ocean water varies, what are a couple possible reasons for these changes? 31. Why do many deep sea fish camouflage themselves with red or orange pigmentation? 32. What piece of equipment do oceanographers use to measure the attenuation of light through the water column? List some advantages of this piece of equipment. List some disadvantages.