Review Guide for Members
advertisement

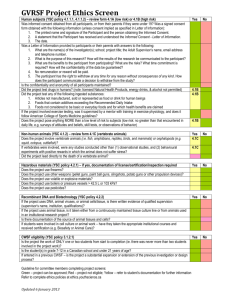
REVIEW GUIDE FOR BELLBERRY HREC MEMBERS It is the responsibility of all Bellberry HREC Members to familiarise themselves with each study Protocol and other supporting documentation and consider whether the proposed research meets the Values and Principles of Ethical Conduct detailed in Section 1 of the National Statement. Two themes must always be considered in human research: the risks and benefits of research, and participants’ consent (National Statement Section 2). The Human Research Ethics Committee conducts an independent review of research studies. Their function is: To decide whether each research project reviewed meets the requirements of the National Statement on Ethical Conduct in Human Research (2007) (National Statement) and is ethically acceptable. To ensure that all research projects are reviewed in accordance with the National Statement. The National Statement is based on values; respect for human beings, research merit and integrity, justice and beneficence which help to shape the relationship between researcher and participant as one of trust, mutual responsibility and ethical equality. To protect the welfare, dignity, rights, safety and well-being of all participants in research projects it reviews. To consider local customs, cultural and social attitudes when evaluating research proposals. To ensure appropriate informed consent is in place for any research project with particular regard to those participants with reduced capacity to consent. To confirm the legitimacy, experience and integrity of the investigator. To provide independent, competent and timely review and monitoring of research projects. The Committees must be satisfied that the above values have been addressed and met and that the following main points have been considered: Scientific design and conduct of the study. The ethics committee should be satisfied that the risk benefit analysis is acceptable and should also consider the impact on the safety of participants’ in the design of the study. Recruitment of research participants. The ethics committee should examine how participants are recruited. Protection of research participants. The ethics committee must look at how the study positively or negatively affects participants or their communities. Informed consent. The ethics committee must decide if the consent forms and process are adequate. Review Bellberry policies, procedures and Terms of Reference for further guidance. Participant Information Sheet and Consent Form (PIS) (National Statement on Ethical Conduct in Human Research 2007 Chapter 2.2). A Participant Information Sheet must accompany each Consent Form. It must not replace personal communication between the investigator and the participant. The Participant Information Sheet is one method of providing information to potential participants to enable them to reach an informed decision regarding their involvement in the research. When preparing your comments, consider whether your concern is related to one of a personal choice of words, or whether the existing wording may have a material impact on the understanding of the study for the participant. Comment Format It would be appreciated if you could use the following format when submitting comments. This will help provide clarity and consistency for the PI: [Document type] [page]/[of number of pages in document] : For example: Protocol 3/123 : The protocol…. Questionnaire 1/2 : Please clarify… PIS 1/12 : Under section…. If there is more than one PIS, please distinguish which PIS the comment relates to. For example use the format PG PIS 1/12 : Under section… Optional PIS 1/12 : Under section… Common Comments: The following may be used as the basis for common questions. These are intended as a guide only, and can be edited as needed. Please add the appropriate document reference at the beginning (per Comment Format above). If you have further suggestions for comments to be added to this list, please email Cathy Stevens on cathystevens@bellberry.com.au Please note this document will be updated from time to time. Please regularly check the Committee Members pages of the Bellberry website for the latest version, as there may be changes to some sections as a result of policy updates WHEN COPYING COMMENTS FROM THIS WORD DOCUMENT AND PASTING INTO EPROTOCOL, ANY APOSTROPHES AND QUOTATION MARKS WILL CREATE A RANDOM SYMBOL ONCE PLACED IN EPROTOCOL. PLEASE CHECK FOR AND CORRECT ANY UNUSUAL SYMBOLS THAT ARE CREATED PRIOR TO SUBMITTING YOUR COMMENTS. WE APOLOGISE FOR THIS SYSTEM GLITCH. Australian Law Clause: The Committee’s legal opinion is that this is an Australian trial and should be governed by Australian Laws. If any information goes overseas then it should not be exempted from Australian Law, and any additional protection should apply. Bellberry approval The Bellberry Human Research Ethics Committee has reviewed this study in accordance with the National Statement on Ethical Conduct in Human Research (2007). Should you wish to discuss the study or view a copy of the Complaint procedure with someone not directly involved, particularly in relation to matters concerning policies, information or complaints about the conduct of the study or your rights as a participant, you may contact the Committee chair, Bellberry Human Research Ethics Committee on 08 8361 3222. Compensation Clause for NonSponsored Trials: POLICYI014 Compensation Clause for Sponsored Trials POLICYI014 Sample Compensation for Injury Clause for Participants For Non-sponsored Trials If, as a result of your participation in this study, you become ill or are injured, immediately advise your study doctor of your condition. In the first instance your study doctor will evaluate your condition and then discuss treatment with both you and your regular treating doctor. Since you are participating in a non-sponsored trial any question about compensation must initially be directed to your study doctor who will advise their insurer of the matter. It is the recommendation of the independent ethics committee responsible for the review of this trial that you seek independent legal advice. Sample Compensation for Injury Clause for Participants If you are injured as a result of your participation in this trial you may be entitled to compensation. Sponsors of clinical trials in Australia have agreed that the guidelines developed by their industry body, Medicines Australia, will govern the way in which compensation claims from injured participants are managed by sponsors. However, as guidelines, they do NOT in any way dictate the pathway you should follow to seek compensation. The sponsor is obliged to follow these guidelines. These guidelines are available for your inspection on the Medicines Australia Website (www.medicinesaustralia.com.au) under Issues/Information – Clinical Trials – Indemnity & Compensation Guidelines. Alternatively, your study doctor can provide you with a hard-copy of the guidelines. It is the recommendation of the independent ethics committee responsible for the review of this trial that you seek independent legal advice before taking any steps towards compensation for injury. Complementary medicines Wherever participants are asked about their medications, there should be an additional statement that this includes complementary medicines and vitamin supplements. Consent Form (more information required) ….Please refer to the Bellberry Sample Consent Form (www.bellberry.com.au) De-identified data: The National Statement avoids the term ‘de-identified data’ as its meaning is unclear. While it is sometimes used to refer to a record that cannot be linked to an individual (‘non-identifiable’), it is also used to refer to a record in which identifying information has been removed but the means still exist to re-identify the individual. Please clarify which of these meanings is intended. GP notification Please include in the consent that “I consent to my GP being notified of my participation in this study and of any clinically relevant”. HIV/Hep C Counselling Please indicate that counselling will be provided both before and after HIV/Hep C testing. HIV Reproductive Risks: Please include the statement - As you are HIV-infected, the use of a condom to reduce the risk of pregnancy and /or the risk of transmission of HIV when having sexual intercourse must occur. Ionising Radiation POLICYI007 As part of every day living, everyone is exposed to naturally occurring background radiation and receives a dose of about 2 to 3 millisieverts (mSv) each year. The effective dose from this study is about (insert number) mSv. (Insert following paragraph 1, 2, 3, or 4) Choose one of the following paragraphs to be inserted above as indicated: 1. Effective doses less than 2 mSv- At this dose level, no harmful effects of radiation have been demonstrated and the risk is negligible or 2. Effective doses between 2 and 20 mSv - At this dose level, no harmful effects of radiation have been demonstrated and the risk is low. The dose from this study is comparable to that received from routine diagnostic medical x-ray and nuclear medicine procedures or 3. Effective doses between 20 and 50 mSv – The dose from this study is comparable to that received from several computed tomography (CT) x-rays procedures. The benefits from the study should be weighed against the possible detrimental effects of radiation, including an increased risk of cancer. In this particular study, the risk is moderate and the theoretically calculated risk of contracting fatal cancer in the future is considered acceptable. The possible detrimental effects of radiation should be weighted against the benefits from the study which are (state benefits to the participant or to society). If there are no perceived benefits from participating then state this. or 4. Effective doses greater than 50 mSv - This research study involves exposure to a significant amount ionizing radiation. The benefits from the study should be weighed against the possible detrimental effects of radiation, including an increased risk of cancer. In this particular study, the risk is moderate and the calculated risk of such harm is about 1 in.... (Calculate using the ICRP risk coefficient for fatal cancer in the general population of5 x 10-2 per Sv. For studies in children or for persons over the age of 50, the risk of radiogenic cancer should be calculated using age- and sex-specific risk factors. The possible detrimental effects of radiation should be weighted against the benefits from the study which are (state benefits to the participant or to society). If there are no perceived benefits from participating then state this. You will be given a certificate that states the radiation dose you have received from participating in this study. You should keep the certificate for five years and show it if you are recruited for any other research studies in that time. Medical Record Access by Bellberry Where the PIS indicates Bellberry has access to medical records: …Please amend “medical records” to “study records” Medicines Australia website: The hyperlink to Medicines Australia guidelines is incorrect. Please replace with "These guidelines are available for your inspection on the Medicines Australia Website (www.medicinesaustralia.com.au) under Issues/Information – Clinical Trials – Indemnity & Compensation Guidelines. Alternatively, your study doctor can provide you with a hard-copy of the guidelines.” Participant Complaint Mechanism The Bellberry Human Research Ethics Committee (A, B, C or D) has reviewed and approved this study in accordance with the National Statement on Ethical Conduct in Human Research (2007). Should you wish to discuss the study or view a copy of the Complaint procedure with someone not directly involved, particularly in relation to matters concerning policies, information or complaints about the conduct of the study or your rights as a participant, you may contact the Chair, Bellberry Human Research Ethics Committee on (08) 8361 3222 or Email: bellberry@bellberry.com.au Participant Payment Details of the payments in situations where participants withdraw, or, are withdrawn for non compliance or other reasons must be described. Pro rata payment should be made to all participants in all circumstances except where they are withdrawn due to an adverse event. In this case they should receive full payment for their participation. POLICYI002 Participants Please replace all references to “subject/s” with “participant/s” per National Statement Section 1. Pregnancy (For Women) Sample Clause for Participants and Partners of Participants USE ONLY RELEVANT SECTIONS AS APPROPRIATE FOR EACH STUDY POLICYI013 Because the experimental agents in this study may affect an unborn baby, you should not be pregnant or become pregnant while on this study and for …… months following the end of the study. You must confirm to the investigator that, to the best of your knowledge, you are not pregnant now, and that you do not intend to become pregnant during the study. You must use an accepted form of contraception and if currently lactating, you should not breast feed your baby while on this study and for 3 months after the last dose of study drug has been taken. Examples of highly effective methods of birth control are: (1) double barrier birth control including oral contraceptive or intrauterine device (IUD) together with a condom, or (2) a diaphragm with spermicide together with condom. If you are uncertain of what form of contraception is acceptable for use during the study, then please ask your study doctor. If you suspect that you have become pregnant during the study, you must notify your study doctor immediately. You will not be able to continue participation in the study if you become pregnant. In the event you do become pregnant the Sponsor will request that you sign a separate consent form to allow monitoring of your pregnancy and the birth and the health of your child up to ……..years of age. Pregnancy (For men) POLICYI013 Sample Clause for Participants and Partners of Participants USE ONLY RELEVANT SECTIONS AS APPROPRIATE FOR EACH STUDY Because the experimental agents in this study may affect an unborn baby, you should not father a baby while on this study and for …… months following the end of the study. The effect the drug has on your fertility may not be known. It is recommended that a condom be worn for all sexual relations as the study medication may affect your sperm risking the potential for an abnormal child being born. It is also highly recommended that you inform your partner of your participation in the study and that contraception has been strongly recommended. Further, you must agree that if your partner becomes pregnant while you are on the study, you will advise the study doctor who will then provide you with an authorisation form to present to your partner. If she is in agreement, that authorisation will function as consent to approve the study doctor’s access to medical information to allow monitoring of the pregnancy, and the birth and the health of the child up to ………years of age. Registering the Trial: Before beginning the clinical phase of the research, researchers should register clinical trials in a publicly accessible register (National Statement 3.3.12). Please comment on why the project is not registered. Seville Oranges Seville oranges are a very sour citrus similar to grapefruits. Some research has shown that Seville oranges contain dihydroxybergamottin which can reduce or increase the activity of some drugs. Sexual Activity and Medication Risks It is recommended that a condom be worn for all sexual relations as the study medication may cause harm to your partner through the absorption of the study drug from the seminal fluid. POLICYI013 Tissue Donation Tissue Donation:- (All discussion of tissue donation must be provided in this section only, using one of the following statements as appropriate.) i. We are requesting the use of a sample of blood/tumour/etc for studies related to how this drug works. This will require……(provide details such as blood sample, extra biopsy). It is optional/non-optional. (If optional there must be a statement on the consent form with check boxes to tick for opt in or opt out.) ii. We are requesting the use of a sample of blood/tumour/etc for storage for future studies that may or may not be related to your condition or the drug used in this trial. This will require……(provide details such as blood sample, extra biopsy). The donation of tissue for this purpose is optional and is covered in a separate Information Sheet and Consent Form. Per Bellberry Sample Participant Information Sheet (www.bellberry.com.au) Typographical errors Numerous typographical errors were noticed throughout the documents. Please review and correct. e.g……….. General reference to Bellberry clauses/ policies/ procedures ….Please refer to the Bellberry sample… (www.bellberry.com.au)