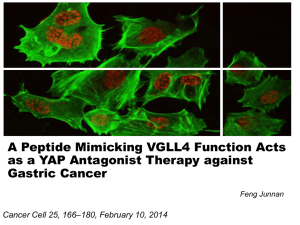
Chapter 3. Regulators of the Hippo pathway core components
advertisement

0 Laymen’s summary In multicellular organisms control over organ size and shape is of utter importance. However, the regulation of control is rather complex. An involved signalling route is the Hippo pathway. In this review, the core cascade and downstream target of the Hippo pathway will be discussed. The first components of the pathway, Warts, Salvador, Hippo and Mats, were discovered in a search for tumour suppressors in the fruitfly (Drosophila melanogaster). The downstream target is the transcription co-activator Yorkie (YKI), which targets many genes implicated in proliferation and apoptosis. These processes are involved in growth control. Deregulation can, therefore, result in severe effects, such as tumourigenesis. Furthermore, the upstream regulators of the Hippo pathway will be disclosed. Various factors are shown to regulate the Hippo pathway core kinases and/or Yorkie. The vastness of the upstream regulators is required for tissue, timing and organism specificity. In order for the Hippo pathway to affect the overall tissue, communication between cells is necessary. Therefore, cell-cell interactions are required to tightly control and modulate Hippo activity between neighbouring cells. Cross talk between the Hippo pathway and other mechanisms is necessary to coordinate the complex development and homeostasis of tissue size, shape and patterning. Taken together, the Hippo pathway is important for processes, such as proliferation and apoptosis. Even though, the regulation of the pathway is understood more and more, research is required to elucidate the signalling route even further. Since deregulation of the pathway can result in tumour formations, better understanding of the pathway could help better understand tumour development and progression. Moreover, insights in the signalling route might help develop therapeutic targets for cancer and other diseases. 1 Contents ABSTRACT 3 INTRODUCTION 4 CHAPTER 1. THE DISCOVERY OF THE CORE COMPONENTS OF THE HIPPO PATHWAY 6 CHAPTER 2. DOWNSTREAM TARGET OF THE CORE CASCADE OF THE HIPPO PATHWAY 10 CHAPTER 3. REGULATORS OF THE HIPPO PATHWAY CORE COMPONENTS 15 CHAPTER 4. CELL-CELL INTERACTIONS INFLUENCE THE REGULATION OF THE HIPPO PATHWAY 22 DISCUSSION 28 APPENDIX 33 2 Abstract In multicellular organisms control over tissue/organ size, shape and patterning is of utter importance. However, the regulation of control is rather complex. An involved signalling route is the Hippo pathway. In this review, the core cascade and downstream target of the Hippo pathway will be discussed. The first components of the pathway, Warts, Salvador, Hippo and Mats, were discovered in a search for tumour suppressors in Drosophila melanogaster. The downstream target is the transcription co-activator Yorkie (YKI), which targets many genes implicated in proliferation and apoptosis. These processes are involved in growth control. Deregulation can, therefore, result in severe effects, such as tumourigenesis. Other processes influenced by Yki targeting are cellcycle exit, differentiation, polarization, migration and metabolism. Furthermore, the upstream regulators of the Hippo pathway will be disclosed. Various factors are shown to regulate the Hippo pathway core kinases and/or Yorkie. The vastness of the upstream regulators is required for tissue, timing and organism specificity. In order for the Hippo pathway to orchestrate effects in the overall tissue, communication between cells is necessary. Therefore, cell-cell interactions are required to tightly control and modulate Hippo activity between neighbouring cells. Cross talk between the Hippo pathway and other mechanisms is necessary to coordinate the complex development and homeostasis of tissue size, shape and patterning. Taken together, the Hippo pathway is important for processes, such as proliferation and apoptosis. Even though, the regulation of the pathway is understood more and more, research is required to elucidate the signalling route even further. Since deregulation of the pathway can result in tumour formations, better understanding of the pathway could help better understand tumour development and progression. Moreover, insights in the signalling route might help develop therapeutic targets for cancer and other diseases. 3 Introduction Tissue and organ size control is an important and complicated process in multicellular organisms. On a single cell level, size can be controlled by cellular growth. Mass accumulation is mostly regulated by synthesis of proteins and macromolecules. Proliferation can decrease cell size in that the mass is distributed over two cells1. However, size control is more intricate in tissue respect. Here, division does not negatively affect overall size, since division does not affect the overall size of the tissue. Rather, proliferation positively affects tissue size, because an increase in cell number allows a larger number of cells to also increase in size, eventually leading to tissue growth2. Tissue size, is therefore, controlled in part by proliferation and is negatively regulated by processes such as apoptosis1, 3-7. Cells have their own growth properties, which thus need to be coordinated in order to elicit size, shape and patterning on a whole tissue scale. In other words, communication between cells in a tissue is of importance for cells to regulate tissue size2. The effects of proliferation and apoptosis, therefore, need not be programmed (intrinsic), but dynamic; adapting to changing environmental signals. There is a need for growth allowing conditions, such as sufficient space, nutrient availability and an absence of stress factors. For communication, however, signal transduction, via cell-cell interactions in the tissue, is required2. Furthermore, a mechanism/integrator regulating growth in response to these various signal inputs is required. One such mechanism identified in the recent years is the Hippo pathway. The Hippo pathway has been shown capable of transducing outside signals to affect gene expression and consequently influence tissue size, shape and patterning. The first components of the Hippo pathway were discovered in a screen for tumour suppressors in Drosophila3, 5-9. The identification of components in such a screen did not come as a surprise, since regulators involved in growth control, could well affect tumour development and progression. In fact, the Hippo pathway was seen deregulated in various cancer cell lines and hyperproliferative growths in vivo1, 3, 5, 6, 10-15. These studies show that the deregulation of the Hippo pathway often leads to hyperproliferation and might even result in tumourgenesis. However, the role of the Hippo pathway seems to encompass more than the modulation of proliferation and apoptosis alone. Studies have identified its involvement in differentiation, cell-cycle exit and migration as well14, 16-18. As explained in the ‘Hallmarks of Cancer19’, development and progression of cancer is dependent on deregulation of several processes. For tumour development an important property is the capability of inducing and sustaining growth. Furthermore, programs, which negatively regulate cell proliferation, such as apoptosis, need to be circumvented. In tumour progression processes, such as the deregulation of polarity and stimulation of migration/invasion, are also involved. In this review, the known characteristics of the function of the Hippo pathway will be discussed to help understand its frequent involvement in tumour development and progression. Here, the identification of the Hippo pathway core components, Hpo, Wts, Sav and Mats, will be discussed (chapter 1). Furthermore, regulation and function of the most known downstream regulator, Yki/Yap, a transcription co-activator targeting many genes, will be disclosed (chapter 2). The extensive upstream regulators of both the Hippo pathway core components and Yki/Yap, will be reviewed as well (chapter 3). Lastly, the ‘communication’ aspect, consisting of input signals and transducers involved in Hippo signalling, will be considered (chapter 4). Taken together, the extent and complexity of the pathway will become clear. As mentioned previously, intrinsic 4 regulation of cell growth control does not impact on tissue scale, therefore, better understanding of the regulation on tissue scale will become more clear when focussing on all interacting aspects. Regulation on tissue scale requires ‘communication’ between neighbouring cells in which many factors and mechanisms/pathways are involved. The importance of the mechanism is further emphasized by the conservation of many of the core components (Table 1, appendix). The complexity of the mechanism seems related to differing functions in timing, tissues and organisms. Overall, the pathway is complex due to the various regulatory factors and targets, cross talk with other mechanisms and feedback. There is no one way to describe the function and mechanism of action. Even though, understanding of the pathway has increased enormously, much remains elusive. The Hippo pathway is known to be important in tissue/organ development and homeostasis of size, shape and patterning. Deregulation of the pathway can result in severe defects, such as cancer. However, it is also implicated in other diseases, such as obesity, immunity and organ deformations 14, 15, 2022. Further research is required to elucidate the mechanism of tissue/organ development and homeostasis control. Better understanding of the Hippo pathway might help better understand the process underlying tumour development and progression. Consequently, it could help in the development of therapeutic targets for cancer and other diseases. 5 Chapter 1. The discovery of the core components of the Hippo pathway Even though, the pathway is often called the Hippo pathway, the first discovered component of the pathway was in fact not Hippo9. In the mid nineties new screens in the model organism Drosophila melanogaster were developed to facilitate the discovery of new tumour suppressors. Many tumour suppressors in this organism were previously found using homozygous mutants. However, several mutations in tumour suppressors are embryonic lethal, making other more sensitive methods to study and discover them necessary. One of the newer methods emerging at these times was the use of mosaic organisms. This method proved an efficient system in that it is analogous to that of tumour growth in humans. In patients usually only minor groups of cells are mutated and cancerous amongst a majority of wildtype cells. In mosaic organisms a comparable situation is present of mutated groups of cells amongst wildtype cells. Furthermore, the wildtype and mutated groups of cells can easily be compared to detect phenotypic differences, such as proliferation rate and morphology, in screens. The genetic mosaic in Drosophila could be induced using different arising techniques. The use of the FLP/FRT recombination system, a site-specific mitotic recombination system adapted from yeast, gave rise to the discovery of the first component of the Hippo pathway1, 2. The mosaic screen for highly proliferative phenotypes performed by ‘Xu T., et al., 19959’ led to the identification of the tumour suppressor Warts (Wts/Large tumour suppressor/Lats). The gene encodes a serine-threonine kinase. Sequence comparisons showed that it has a sequence similarity to three proteins involved in the regulation of cell-cycle control, cell growth and proliferation in the budding yeast Neurospora. This similarity further emphasised the possible tumour suppressor function. Later on, the human homologues Lats1 and Lats2 were found, indicating a high degree of conservation in function and sequence8, 23. Since the initial use of FLP/FRT recombination in Drosophila by ‘Xu T., et al., 19959’ in mosaic screens, several other tumour suppressors were discovered. It was not until seven year later, though, that another component of the Hippo pathway was discovered1, 6. The mosaic screens performed in Drosophila became more refined by focusing on proliferation differences in the compound eye. Subtle changes in proliferation and morphology were easily detected when comparing the size and arrangement of specifically shaped ommatidia. The development of the complex compound eye is tightly regulated by both cell proliferation and apoptosis1, 5. This focus on both processes led to an important discovery. A mosaic screen by ‘Tapon N., et al., 20021’ gave rise to the identification of, Salvador (Sav/Shar-pei), a novel tumour suppressor involved in both processes. To this point relatively few tumour suppressors, active in both proliferation and apoptosis, were known. Sav mutants showed both elevated levels in cyclin E (CycE) and Drosophila inhibitor of apoptosis 1 (DIAP1). The prior protein is required for the modulation of cell-cycle exit. The down-regulation of cycE has been shown to induce cell-cycle exit and limit proliferation subsequently24. Reversely, the increase of protein levels resulted in a higher degree of proliferation. The latter protein, DIAP1, is involved in apoptosis1, 3. This protein is capable of inhibiting apoptosis by binding to caspases. An up-regulation will thus result in holding off apoptosis25. The tumour suppressor function of Sav was stressed even more by further research. Sav proved to be highly conserved from C. 6 elegans to humans. Moreover, the human orthologue was mutated in three cancer cell lines1, 5, 6. The fact that Sav is a component of the Hippo pathway was discovered when looking at a molecular level. The protein contained a C-terminal coiled-coil region and a N-terminal WW domain. Proteins containing WW domains are usually capable of protein-protein interactions. The use of double mutants and GST pull-down assays revealed the fact that Sav is capable of interacting with Wts1, 5. Further experimentation proved this interaction and a scaffolding function for Sav was proposed1, 5, 26. Short after the discovery of Sav, five individual papers mentioned the discovery of another component of the pathway: Hippo (Hpo) itself3, 4, 26-28. Again, the tumour suppressor, which encodes a serine-threonine kinase part of the Sterile 20 family, was discovered in mosaic screens. In these screens mutations that resulted in overproliferation, an increase in cell growth and impaired apoptosis were selected. Figure 1. shows the overgrowth phenotype in hpo loss of function mutants3. These results are similar with the above mentioned wts and sav loss of function mutant phenotypes (data not shown). Since, both cycE and diap1 were up-regulated in hpo mutants as well, there was an indication that there could be a connection with Wts and Sav3, 4, 26-28. Several interaction and binding assays proved that there was in fact a physical connection. Yeast two-hybrid experiments using both Hpo and Sav as bait showed both proteins could interact3, 4. Furthermore, binding assays showed that it was in fact the C-terminal coiled-coil region of Sav that was capable of binding the Cterminal region of Hpo. Hpo, then, stabilises Sav by phosphorylation 3, 4, 26-29. Binding assays showed that there was a physical interaction between Hpo and Wts as well. However, it is not the C-terminal, but the N-terminal region of Hpo that is necessary for the interaction with Wts3, 27. The catalytically active N-terminal region of Hpo is able to phosphorylate and activate Wts3. Interaction of Hpo with Sav facilitates this phosphorylation. Furthermore, genetic epistasis showed Hpo is active downstream of Sav, but upstream of Wts in the pathway11. In contrast to the other components of the pathway, Hpo is able to affect the process of apoptosis directly. Hpo is able to directly phosphorylate DIAP1 in order to induce its subsequent degradation4, 28. Furthermore, Hpo can induce the expression of the pro-aptotic protein Head involuted defect (hid), which can bind and inhibit DIAP11, 4. 7 Figure 1. Overgrowth effects in hpo loss of function clones in Drosophila melanogaster. (A-F) Scanning electron micrographs (SEM) of wildtype (A) and hpo mutant clone containing (B-F) Drosophila tissue. (A-B) Show the fly head. (C-D) Show a side view of the compound eye. (D) Shows a less severe hpo mutant. (E) Shows the fly notum. Dashed lines outline the hpo mutant clones. (F) High magnification of the epidermal cells. Dashed lines show the border between wiltype (left) and mutant (right) clones. (G) Drosophila wing tissue containing hpo mutant clones. Mutant clones are outlined by red dashed lines. (H) Drosophila leg portion containing hpo mutant clones. Mutant clones are outlined by red dashed lines. (I) Section of adult Drosophila eye tissue containing wildtype (upper right) and hpo mutant clones (lower left). Boundary between clones is marked by red dashed lines. Wildtype clones contain pigment. Note the increased spacing in mutant clones between mutant photoreceptor clusters, due to an increase in interommatidial cells (data not shown). Results from Wu, S., et al., 20033 (check to see used genotypes produced by FLP/FRT recombinase). So far, three of the core components of the Hippo pathway were known. The interaction of the scaffolding protein Sav with the kinase Hpo is needed to facilitate the phosphorylation of the kinase Wts. Nonetheless, it was still unknown how the catalytic activity of Wts was required for the modulation of proliferation and apoptosis. Other mosaic screens in the compound eyes of Drosophila were performed in hopes of finding a phosphorylation target of Wts. The screen performed by ‘Lai Z.C., et al., 20057’ led to the identification of the tumour suppressor Mats. Mats encodes a non-catalytical protein, part of the Mob superfamily7, 30, 31. However, subsequent binding assays showed the protein was not a Wts target for phosphorylation. In fact, Mats interacts synergistically, as an activating subunit, with Wts. The protein is functionally and genetically conserved in mammals. Furthermore, its tumour suppressor function is emphasised by the fact that the gene is mutated in mammalian cancer cells7, 30, 31. More resent studies on the mammalian orthologues Mob1A and Mob1B showed that Mst1 8 and Mst2 (Hpo orthologues) phosphorylate Mob in order to enhance its ability to bind Lats1 (Wts orthologue) 31. Similar to the previously discovered components of the Hippo pathway, Hpo itself is evolutionary conserved with human orthologues Mst1 and Mst2. Even the phosphorylation of Wts homologues Lats1 and Lats2 by Mst2 through the catalytic Cterminal conserved29. The high degree of physical and functional conservation of many of the pathway components indicate a tissue size control mechanism which might be universal in all organisms3, 26-29. All in all, the use of mosaic screens to identify new tumour suppressors led to the discovery of the four core components of the Hippo pathway (Figure 2). Hpo binds and phosphorylates the scaffolding protein Sav, which is consequently stabilised. The interaction between Hpo and Sav is necessary to facilitate the interaction between Hpo and Mats and Wts. Hpo is able to phosphorylate Mats in order to enhance the ability of the latter to bind Wts. The binding of Wts to Mats is needed for Hpo to phosphorylate and activate Wts. Eventually; the activation of the pathway will result in the modulation of proliferation and apoptosis. To this point, the direct downstream targets of these core components remain unknown, though. In the next chapter, the discovery of the direct target of Wts will be discussed. Figure 2. Schematic overview of the Hippo pathway core kinases in Drosophila. Hpo interaction with the scaffolding protein Sav is required for Hpo phosphorylation activity. The interaction between Sav and Hpo is stabilized by Sav phosphorylation. Hpo phosphorylates the downstream targets Mats and Wts. Mats functions as a scaffolding protein required for the activity of Wts. Phosphyralation by Hpo stabilizes the complex and activates Wts. Wts negatively regulates proliferation and levels of CycE and DIAP1 via a downstream target. Hpo can directly modulate DIAP1 levels. Human Hippo pathway regulation is fairly similar, apart from Cyclin E transcription. Conserved mammalian genes are shown in Table 1, appendix. 9 Chapter 2. Downstream target of the core cascade of the Hippo Pathway Previous studies have shown that the Hippo signalling pathway is involved in the regulation of tissue size; tightly regulating proliferation as well as apoptosis. Several genes, cycE and diap1, implicated in proliferation and apoptosis are involved, albeit indirectly. The exact manner by which the tumour suppressor core components of the pathway elicited the size control remained unclear. It was not until a Yeast Two-Hybrid experiment was performed, mentioned in ‘Huang J., et al., 200510’ (Figure 3), that a target for the core components was discovered. In this experiment Wts was used as bait resulting in the detection of an interacting protein. The Drosophila protein, later named Yorkie (Yki), showed a 31% identity with is human orthologue Yes-associated protein (YAP), which is a transcription co-activator. The functional conservation is emphasised by the fact that Yap can rescue the phenotype the yki loss of function mutants10. Further in vitro binding assays and in vivo kinase assays showed that Yki could indeed associate with Wts and was phosphorylated upon its association10, 15. This interaction required the WW domains in the C-terminal region of Yki and the non-catalytical N-terminal region of Wts10. In vivo, Yki overexpressing clones showed overgrowth phenotypes similar to that of loss of function hpo, wts or sav mutants. Furthermore, overexpression of Yki led to an increase in expression of DIAP1 and CycE, showing Yki activation is involved in increased transcription of diap1 and cycE. Epistatic analysis led to the inclination that Yki functioned downstream of the Hippo signalling cascade; Yki being in fact inhibited through the phosphorylation by Wts10, 15. Figure 3. Yeast Two-Hybrid experiment showing Yki is capable of binding Wts. Sos-Wts is used as bait for library screening. Sos is an empty bait containing Sos only. Myr is used as control prey, containing myrosylation signal only. 18-418, 186-418 and 229-418 are three independent Yki clones isolated from the screen. The number represents the starting and ending position of the Yki polypeptides in the clones. 25°C is used as control temperature, whereas 37°C shows the results of the Y 2-H screen. Results from 43 Elucidation of the function of Yki was made possible by looking at its subcellular localisation, using epitope tagged proteins and antibody staining11, 15, 33. Yki localises to both the cytoplasm and nucleus in absence of the Hippo pathway core components. Coexpression of these components localises Yki less to the nucleus and more to the cytoplasm. As a transcription co-activator this re-localisation is inhibiting its function, since its transcription co-activating function can only be performed inside the nucleus. 10 There are other transcription regulators known to shuttle to the cytoplasm. Many of these proteins are shown to interact with the cytoplasmic localisation protein, 14-3-315. Prior to the discovery of the involvement of Yki in the Hippo pathway, its human orthologue Yap was found accumulated in the cytoplasm as a complex with 143-334. The interaction between Yap and 14-3-3 suggested an interaction between Yki and 14-3-3 was possible as well. Furthermore, Taz, another human orthologue of Yki, was known to be able to bind 14-3-3 upon phosphorylation of a single serine residue of a certain motif34. This motif is conserved at S127 in Yap and at S168 in Yki. In vivo experiments show the phosphorylation of these residues was found necessary to induce cytoplasmic Yap/Yki localisation and sequestration. Mutating either S127 or S168 to a non-phosphorylatable variant phenocopies both Yki overexpression and hpo, wts or sav loss of function mutants15, 32, 33. Therefore, binding to 14-3-3 keeps Yki in the cytoplasm. The phosphorylation of the S168 residue and subsequent sequestration of Yki by 14-3-3 is not sufficient to explain all the effects of the Hippo pathway on Yki though15, 32, 33, 35, 36. Other mechanisms are involved in the inhibition of Yki. Experiments, using nonphosphorylable mutants encompassing more than the S168 region (Yki: V53SA) showed more dramatic overgrowth effects, indicating the importance of other regions33, 36. Within this larger region reside the two other speculated phosphorylation sites S111 and S250. Experiments, using Phos-tag gels, phenotype screens and subcellular localisation assays, showed that these sites can indeed be phosphorylated by Wts, resulting in inhibition and cytoplasmic localisation of Yki36. However, the inhibition via phosphorylation of these sites is independent from Yki binding to 14-3-337. Moreover, Yki:V53SA alone showed more overgrowth than with co-expression of components of the Hippo pathway, indicating the presence of an inhibition mechanism which is not dependent on phosphorylation. This indication is strengthened by the reduced nuclear localisation when Hpo and/or Wts were co-expressed38. Another tumour suppressor, Expanded (Ex), which will be looked at more in depth in the next chapter, showed a similar Yki inhibitory function independent of phosphorylation. Affinity chromatography coupled with mass spectrometry showed that Yki is in fact a direct binding partner of Ex35. The direct binding of Ex, Hpo and Wts seems dependent on the WW domain of Yki and the PPXY motifs present in Ex, Hpo and Wts35, 38. All in all, many phosphorylation dependent and independent processes are involved in the inhibition of Yki. The greatest inhibiting influence on Yki is via its phosphorylation at the S168, though. The alteration is necessary to facilitate Yki binding to 14-3-3, which shuttles and sequesters Yki from the nucleus to the cytoplasm35, 36, 38. Furthermore, the importance of the phosphorylation dependent inhibition of Yki emphasises the necessity of regulation via the Hippo pathway. Next, the function of Yki in relation to proliferation and apoptosis regulation will be explained. As mentioned previously, the overexpression of Yki resulted in an overgrowth phenotype. The cause of this phenotype was the transcriptional up-regulation of several genes with proliferative and anti-apoptotic functions. Among these genes in Drosophila cycE and diap1, but also dMyc have been shown to be a Yki target1, 3, 10, 15, 24, 25, 39. Furthermore, bantam miRNA is discovered as a Hpo pathway transcription target. The bantam miRNA targets both proliferation and apoptosis, phenocopying Yki overexpression40, 41. In mammals, several similar targets, including some diap1 and dMyc homologues, are known. Other targeted genes are proliferation-involved genes, encoding proteins such as Ki67, sox4, H19 and AFP, and the anti-apoptotic gene encoding MCL1. However, the up-regulation of Cyclin E1 or Cyclin E2 has not yet been discovered. In Drosophila, the CycE up-regulation is tissue specific, showing elevated 11 levels in the eye imaginal discs and around the morphogenetic furrow only15. The numerous transcriptional targets, which are not present in all tissues or not always conserved, suggest a more tissue specific targeting role for Yki. Nevertheless, knowing Yki as a transcription co-activator, a direct binding partner was still necessary, yet remained elusive. Several transcription factors were speculated to associate with Yap, among these were p73, Runx2, ErbB4 and members of the TEAD/TEF family10, 34. ‘Vassilev, A., et al., 200134’ showed an interaction between human orthologue Yap and the four members of the TEAD/TEF family was possible, before the connection with Yap and the Hippo pathway was made. Subsequent studies focussing on the direct binding partner of Yap found that transcription factors of the TEAD/TEF family were indeed the most important activating binding partners of Yap32, 42. Furthermore, the Drosophila orthologue Scalloped (Sd) was found to be able to bind Yki in several in vitro and in vivo experiments as well13, 43, 44. The C-terminal region of Sd can associate with a conserved N-terminal domain of Yki. The binding of Sd to Yki is independent of phosphorylation38. Upon binding, the Sd-Yki complex localises to the nucleus where transcription of target genes is enhanced13, 34, 43, 44. Interestingly, the association of Sd and Yki is a more complex interaction than it seems. The down-regulation of Sd causes an increase in bantam miRNA expression instead of the expected decrease13. However, mutations of sd phenocopy loss of function mutations in yki, indicating a similar function13, 43, 44. The different effects on the transcription of the target gene bantam miRNA, indicate there might be a more complex mechanism involved. Dosage experiments mentioned in ‘Wu, S., et al., 200843’ further emphasise the delicate interdependence between Yki and Sd. Although, Sd and the TEAD/TEF orthologues might be the most important transcription factor, small phenotypic differences comparing sd and yki mutants indicate it is possibly not the only transcription factor involved13, 43, 44. There are other transcription factors known to interact with Yki/Yap. Both the TALE-homeodomain protein Homethorax (Hth) and the zinc finger transcription factor Teashirt (Tsh) have been shown to interact with Yki and up-regulate transcription41. However, both transcription factors are active only in the eye imaginal discs, further emphasising the possible spatiotemporal mechanism underlying Yki function. Research performed over the years underscore the importance of the Hippo pathway targets in development, tissue size homeostasis and its apparent oncogenic predisposition. The suggestion of Yki/Yap being oncogenic is not surprising. The first indication is the strong overgrowth phenotypes visible in several mutants1-3. Secondly, Yki target proteins are often up-regulated in many cancer cell lines and tumours, for example Myc and IAP family members10, 15, 32. Thirdly, in vivo experiments show short induction of overexpression of Yap in mice result in hepatomegaly (Figure 4). Moreover, longer induction of overexpression results in tumour growth15. However, the discovery of down-regulated Yap in cancer patients indicates that it may not be an oncoprotein. This controversy, together with the above-mentioned questions surrounding the actual mechanism, stresses the complexity of the transcriptional regulation facilitated by Yki/Yap. 12 Figure 4. Effects of Yap overexpression in vivo in mouse livers. Mouse strains were created crossing ApoE/rtTA mice (reverse tetracycline transactivator (rtTA) under control of the liver specific ApoE promotor) with Yap mice (human Yap cDNA driven by minimal CMV promotor and tetracycline response element). The cross resulted in a ApoE/rtTA-Yap strain, in which Yap was expressed upon treatment with the tetracyclin containing drug Doxycyclin (Dox). Treatment consisted of a 0.2 mg/ml Dox dosage starting at 3 weeks of age. (A-B) Show hepatomegaly after 1 week and 4 of Yap overexpression (right) compared to wildtype livers (left). (C-D) Shows prolonged overexpression of Yap, (eight weeks (C) and 3 months (D), leading to tumourigenesis. Arrow heads in (C) point out nodules scattered throughout the liver. Results from Dong J., et al., 200715. Taken together, the tumour suppressive Hippo components are the main regulator of the activity of Yki by inhibiting its function. If not inhibited, Yki can bind possibly several different transcription factors as a transcription co-activator. The most known and supposedly the most important interacting transcription factor is Sd. By binding a transcription factor, Yki can regulate the transcription of several genes involved in proliferative and anti-apoptotic processes. A schematic overview of Yki regulation and signalling is depicted in Figure 5. Having elucidated the downstream mechanism and thus the effect of the Hippo pathway further, the question of the cause of its (in)activation still remains. Hence, the upstream regulation of the Hippo pathway will be discussed in the following chapter. 13 Figure 5. Schematic overview of Yki regulation and signalling in Drosophila. Yki can be phosphorylated directly by Wts. The phosphorylation enables binding to 14-3-3 resulting in cytosolic sequestration. Hpo is also shown to inhibit Yki, however, the specific mechanism remains unknown. Ex is shown to directly bind and inhibit Yki in a phosphorylation independent manner. If active, Yki is able to bind transcription factors such as Tsh, Hth and Sd. Of these transcription factors, Sd is the best understood and possibly the most important. Binding of Yki activates transcription of several genes involved in proliferation and apoptosis. Human Yki regulation and signalling is fairly similar, apart from Cyclin E transcription. Conserved mammalian genes are shown in Table 1, appendix. 14 Chapter 3. Regulators of the Hippo pathway core components The results of many studies over the years have led to the unfolding of a complex and highly regulated network of proteins being involved in the Hippo pathway. A recent study, ‘Kwon Y., et al., 201345’, developed a protein-protein interaction network (PPIN) of the Hippo pathway. The network was created using mass spectrometry, using some of the most described and important existing pathway components as bait. The resulting network comprised 153 different proteins and 204 interactions, revealing the extensiveness of the Hippo pathway. Since, the Hippo pathway is this extensive, only the most known and some of the most recent interacting proteins will be discussed in this chapter. Perhaps the most known proteins regulating the Hippo pathway in Drosophila are Merlin (Mer) and Expanded (Ex). Merlin was already a known tumour suppressor in mammals. The human orthologue of Mer is also known as the neurofibromatosis type-2 (NF2) gene, which gives rise to a familial cancer syndrome in the central nervous system when mutated. Mer is part of the protein 4.1 superfamily of adaptor proteins with a FERM domain. A related protein containing a FERM domain is Ex 12. Binding assays and in vivo localisation shows both proteins can interact and localise to adherence junctions independently of one another12. Mosaic screens showed double mutant mer and ex clones have a tissue overgrowth phenotype, indicating a role in growth control. The overgrowth phenotype, caused by defects in proliferation and apoptosis, is similar to that of the previously mentioned hpo, sav and wts mutations (Figure 6) 12. Furthermore, an up-regulation of CycE and DIAP1 levels was also found in the mutants. These results indicate a possible link between Mer and Ex and the Hippo pathway12. The overexpression of either Ex and/or Hpo in different mutant backgrounds showed that the proteins indeed act in the same pathway. Ex overexpression caused undergrowth phenotypes, which could be rescued by hpo loss of function. However, Hpo overexpression could not be suppressed by loss of either mer or ex. These results show that even though they act in the same pathway, Mer and Ex act upstream of Hpo. Later on, Mer and Ex were found to act upstream of all the Hpo core kinases and Yki as well. A Yki reporter gene assay shows that Mer and Ex can repress the activity of Yki in a similar way as Hpo, Sav and Wts10, 12. Since many components of the Hippo pathway are conserved in vertebrates both genetically as well as functionally, it is likely that Mer and Ex orthologues act upstream of the Hippo core kinases in vertebrates12. Accordingly, the regulation of the Hippo core kinases can involve both Mer and Ex. This is not surprising, since both proteins have been shown to interact. Furthermore, the co-expression of both proteins can enhance the phosphorylation of Wts in cultured cells, while expression of one of the proteins alone has a smaller effect. Therefore, Mer and Ex act synergistically to induce the Hippo pathway, possibly by acting parallel to each other12, 46. 15 Figure 6. Mer and Ex regulate tissue growth in Drosophila mid-pupal retina. The retinae were stained with anti-Discs large (Dlg) antibodies that localize to apicolateral junctions and visualise cell outlines. (A) Shows wildtype retina. (B) Shows mer mutant clone retina. (C) Shows ex mutant clone retina. (D) Shows hpo mutant clone retina. (E) Shows retina with wildtype (red and green staining) and mer;ex double mutant (absence of red and green staining) clones. (F) Shows retina with wildtype (red and green staining) and TSC1 (Tuberous Sclerosis Complex 1) mutant clones (absence of red and green staining). TSC1 is a growth control gene affecting growth via a Hippo pathway independent mechanism. Interommatidial cell number was affected in both mer and ex single mutants. However, only the mer;ex double mutant phenocopies the hpo mutant severity. Phenotypical differences in the TSC1 mutant clone indicate the involvement of Mer or Ex in a distinct growth control mechanism. Results from ‘Hamaratoglu F., et al., 200612. The differences between the proteins are further emphasised when looking at Ex in particular. As mentioned in chapter 2, Ex can function as a Yki binding protein, indicating a dual role of the protein when it comes to influencing the Hippo pathway 35. Ex is able to abrogate the function of Yki, by relocalising it from the nucleus to the cytoplasm. This inhibition of Yki takes place downstream of Wts and in concert with the inhibition via 14-3-335. These results, together with the above-mentioned results, imply that Ex can either function as an upstream regulator of the Hippo core kinases or function as a direct binding partner and inhibitor of Yki12, 35. Looking more in depth at hpo, sav and wts mutant cells, elevated levels of Mer and Ex were visible. This elevation of protein levels was due to derepressed transcription. Apparently, the Hippo pathway contains a feedback mechanism to keep the signalling in a steady state. The activity of the Hippo pathway is tightly controlled in a steady state, which can be influenced either positively or negatively. Overactivation of the Hippo pathway leads to inhibition of Yki and thus decreased transcription of targets including Ex and Mer. When the Hippo pathway is repressed, Yki activity is not inhibited resulting in transcription of targets including the upstream regulators of Hippo pathway12. 16 The feedback mechanism notwithstanding, it can be concluded that Ex and Mer are required for Hpo regulation. Nonetheless, immunoprecipitation experiments showed no direct interaction of Mer or Ex with Hpo or Wts. There was a link missing between the Hippo core kinases and the upstream regulators Mer and Ex 12 and this link was discovered a few years later. RNAi and mosaic screens picked up on a protein, Kibra, showing an overgrowth phenotype. The overgrowth was caused by overproliferation and inhibition of apoptosis, indicating a possible involvement in the Hippo pathway. The phenotypes show less overgrowth compared to mutations in the Hippo pathway core kinases. Rather, the phenotypes are similar to mutations in either mer or ex, implying a similar function 46-48. Immunoprecipitation showed Kibra could interact with both Mer and Ex, increasing the interaction between Mer and Ex 47, 48. This result suggests a scaffolding function for Kibra. Additionally, results from RNAi screens and mosaic screens confirm that Kibra functions upstream of the Hippo pathway core kinases46, 47. In fact, co-expression of all three proteins, Kibra, Ex and Mer, increases the activation of the Hippo pathway, indicating again a synergistic effect 48. Furthermore, kibra is a Yki transcription target just as ex and mer, indicating its role in feedback of the Hippo pathway as well47 . However, a double mutant of kibra and ex shows an overgrowth phenotype which is even more severe than hpo, sav or wts mutants. In these double mutants Yki transcription is up-regulated more than in Hippo pathway core kinase mutants, suggesting that overgrowth via Yki activation can be inhibited dependent or independent of the Hippo pathway. As mentioned above, this could be facilitated by Ex, which can bind Yki directly. Moreover, these double mutant results indicate Kibra might possible be involved in Hippo pathway independent inhibition of Yki as well46. Kibra is shown to co-localise with Mer and Ex. This co-localisation is near the apical membrane, indicating a possible function of transducing signals or information from the extracellular environment6, 7. All three proteins can localise independently of one another. More importantly, this localisation seems necessary for the membrane localisation of the Hippo kinase complex. The loss of Kibra, Ex and Mer results in a decrease of membrane associated Hpo. Furthermore, it is this localisation of the Hippo pathway core kinases, which is suggested to be necessary for its activation48. Apparently, the three proteins function as an apical scaffolding mechanism, necessary for the localisation of the Hippo pathway core kinases. This localisation could be necessary in order to stimulate the relay of external signals to internal regulation of the Hippo pathway48. Other than the interaction of Kibra with Mer and Ex, yeast 2-hybrid experiments show Kibra can directly bind Sav, via the WW domain in Kibra. Co-immunoprecipitation shows that Kibra is able to interact with Hpo in a Sav dependent manner. Continuation of yeast 2-hybrid experiments showed Mer is capable of interacting with Sav and Ex with Hpo, contradictory to results mentioned in ‘Hamaratoglu F., et al., 200612’. Further co-immunoprecipitation confirmed these results. Apparently, many of the upstream regulators can interact with each other and/or interact with downstream Hippo pathway core kinases as well48. All together, the specific mechanism remains unclear, yet the formation of interactions seems required for the enhancement and stimulation of the Hippo pathway. Interestingly, the interactions and importance of different components seems tissue specific. Experiments performed in the eye imaginal discs showed Ex as a more important upstream regulator than Mer and/or Kibra46-48. On the other hand, Mer and Kibra seem more important in the ovaries47, 48. The Hippo pathway core kinases seem to 17 act as a signal integrator receiving information from multiple inputs depending on the tissue type and possibly other factors. However, in order to enhance the view on this tissue specificity understanding of the (trans)membrane factors involved in the Hippo pathway is necessary. These factors and the non-cell autonomous implications will be discussed in the next chapter. The above mentioned interactions and involvedness in the Hippo pathway of Mer, Ex and Kibra are all researched in Drosophila. All three proteins are evolutionary conserved. Although, the functional conservation has not fully been explored, a conservation of mechanism is expected. For instance, the synergistic phosphorylation of Lats 1 and 2 in vitro by co-expression of mammalian Kibra and Mer is comparable to that of the phosphorylation of Wts in Drosophila48. However, the tumour suppressive function of Kibra shown in Drosophila might not be fully conserved in mammals. For example, the knockdown of Kibra in a human cancer cell line causes impaired proliferation and migration instead of the expected increase of proliferation and migration. This suggests Kibra has an oncogenic rather than a tumour suppressive function. Note that in this case, instead of the Hippo pathway being involved; the MEKERK-RSK cascade seems involved49. Taken together, the regulation of the Hippo pathway in mammals might be more complex than in Drosophila. Furthermore, the regulatory function of Ex, Mer and Kibra should be elucidated further in both mammals and Drosophila. In the past years, other factors involved in the regulation of the Hippo pathway core kinases have been discovered as well. Among them are RASSF, STRIPAK and AKT, negatively regulating Hpo. Mutants of the Drosophila orthologue, drassf (Ras associated factor), are viable and show growth defects. The mutants are about 15% lighter than wildtype due to a reduced cell number. Co-immunoprecipitation shows the protein is able to interact with Hpo via the SARAH (Sav, RASSF, Hpo) domain26, 50, 51. Apparently, there is a binding competition between dRASSF and Sav for Hpo, suggesting an oncogenic function for dRASSF. However, dRASSF might still contain a tumour suppressive function as well. Especially when looking at the fact that a RAS1 loss of function results in reduced proliferation and increased apoptosis, which can be substantially rescued by drassf mutation. Therefore, dRASSF seems to have both an oncogenic function via the Hippo pathway as a tumour suppressive function via RAS1 50. In mammals these functions seem to be split over two proteins. The mammalian dRASSF orthologue RASSF1 encodes two proteins, RASSF1A and RASSF1C. The prior protein is shown to interact with and activate Mst 1 both in vitro and in vivo51. The latter protein was shown to have oncogenic properties. Deletion of RASSF1A in mice, while RASSF1C remains intact, causes an increase in spontaneous tumour formation52. Even though, dRASSF showed to be a negative regulator of Hpo, a phosphatase able to directly inactivate Hpo remained unknown. A genome wide RNAi screen identified the Drosophila Striatin-interacting Phosphatase and Kinase (dSTRIPAK) PP2A complex as a possible phosphatase53. Affinity purification coupled to mass spectrometry and co-immunoprecipitation indicated dSTRIPAK could indeed associate with Hpo/RASSF complexes and thereby dephosphorylating Hpo. The striatin family member Cka was necessary for the interaction with Hpo and was at least in part dependent on dRASSF. The association of Hpo with dRASSF prevents Hpo from binding Sav. dSTRIPAK is recruited, subsequently, to dephosphorylate Hpo. The STRIPAK mechanism seems conserved, since orthologues are able to control the phosphorylation levels of Mst 1 and 253. 18 Another negative regulator of the Hippo pathway is Akt, also known as Protein Kinase B (PKB). Akt is involved in cell survival via evasion of apoptosis. The evasion of apoptosis is elicited via different routes, though. A newly discovered route is the Hippo pathway. Upon cleavage, activated Mst can activate c-jun N-terminal Kinase (JNK) mediated apoptosis. Akt can inhibit the cleavage of Mst in vivo and thus inhibit the activation of apoptosis20, 21. Moreover, Hpo is able to inhibit Akt as well. Overexpression of Yki shows an up-regulation of akt mRNA levels, showing Akt is a target-gene of Yki20. This is conserved in a human epithelial breast cancer cell line where knock down of Lats1 results in an up-regulation of Akt dependent on Yap function13. It is interesting to note that Akt signalling is implicated in metabolism. Hence, the cross talk between Akt and the Hippo pathway could be necessary to link the availability of nutrients to growth control13. The link between nutrient sensing and the Hippo pathway is further emphasised by another genome wide RNAi screen. This screen led to the discovery of Salt inducible kinases 2 and 3 (Sik 2 and Sik 3) as negative regulators of Hippo signalling 22. The coexpression of Sik 2 and 3 led to overgrowth phenotypes and up-regulation of ex, similar to when yki is up-regulated. Co-immunoprecipitation revealed the Drosophila Sik’s can interact with and phosphorylate Sav. Upon phosphorylation Sav’s ability to induce Hpo activity is inhibited. The phosophylation site on Sav is not conserved in mammals. However, Sik2 does promote Yap dependent transcription in human cells, indicating it might still be functionally conserved in some other way22. Other than Hpo being regulated, Wts is modulated by several factors as well. Coimmunoprecipitation shows Wts can interact with the large myosin protein Dachs 54. This protein was previously identified as a downstream component of the Fat signalling route, which will be discussed in the next chapter. Epistasis experiments show Dachs functions parallel to Hpo, inhibiting Wts upon interaction. Dachs might function as a scaffolding protein necessary for the assembly of a degradation complex ensuing the binding of Wts. Furthermore, Dachs localises apically in presence of Fat 54. An interacting partner of Dachs, required for the inhibition of Wts, was discovered during a genetic screen. The LIM domain containing protein, Zyxin (Zyx), is shown to interact with Dachs. Loss of Zyx reduces Yki activity and thus tissue overgrowth. Evidently, Zyx positively regulates co-activator Yki, by disabling its inhibitor55. A model is proposed where the stability of Wts is regulated via the localisation of Dachs. When Dachs is localised apically, it is able to interact with Zyx to stimulate its interaction with Wts, thereby inducing its degradation. Interestingly, in mammals, Zyx in implicated in linking effects of mechanical strain to cell behaviour, since it can modulate the cytoskeletal organisation of actin bundles. This provides a possible link for mechanical strain and growth control via gene regulation55. Apparently, external factors such as nutrient availability and mechanical strain are involved in the Hippo pathway. Another factor involved is the apical basolateral cell polarity. Many of the proteins involved have actually been shown to localise apically47, 48. The importance of this polarity is emphasised by the fact that in many neoplastic tumours polarity genes are mutated. Disruption of apical basal cell polarity can lead to uncontrolled cell proliferation of epithelial cells, resulting in an epithelial to mesenchymal transition (EMT). This transition often underlies the transition to cancer56. Among these polarity genes are the conserved tumour suppressors Lethal giant larvae (Lgl), Scribbled (Scr) and Disc large (Dlg) 56-58. Lgl opposes the apical 19 localised aPKC complex, consisting of the atypical Protein Kinase C, par-3 and par-6. Normally, aPKC and Lgl are in a sort of balanced state, inhibiting one another. aPKC is able to interact with and phosphorylate Lgl, excluding it from the cell cortex. In lgl mutants, the polarity and epithelial integrity is often disrupted, increasing proliferation and neoplastic tissue overgrowth. Furthermore, DIAP1 and CycE levels are upregulated. These observations indicate a link with the Hippo pathway. Indeed, depletion of Lgl activates Yki. Localisation assays show that Hpo and dRASSF are mislocalised away from the apical cortex in lgl mutants. aPKC activation mislocalises Hpo and dRASSF in a comparable way as seen in lgl mutants57, 58. Together, these results imply that Lgl is required for the localisation of Hpo and dRASSF and that it can be affected by phosphorylation by aPKC. As mentioned previously, the cortical localisation is required for Hippo pathway activation. The balance, between Lgl and aPKC, can be affected by Scr and Dlg, as a loss of polarity is responsible for a balance shift57, 58. Unexpectedly, in lgl mutant clones, the boundary cells are eliminated via JNK mediated apoptosis. The up-regulation of JNK signalling indicates that the Hippo pathway is involved58-60. Since, lack of lgl would cause the Hippo pathway to be mislocalised and deregulated, another non-cell autonomous mechanism is involved58. Another factor involved in promoting basolateral cell polarity in follicular epithelium is the Sterile 20 family member, Tao, is also known for controlling microtubule dynamics61, 62. However, it has been implicated in modulating the activity of Hpo as well. Co-immunoprecipitation shows it can interact with and phosphorylate Hpo. The protein is conserved since its mammalian orthologue, TAOK3, affects the Mst kinases in a similar way62. Tao forms no complex with the other known upstream regulators Mer or Ex, though. This is not surprising since Tao can control cytoskeletal organisation and polarity via microtubule dynamics, indicating a parallel function. Again there seems to be a link between polarity and growth control61, 62. So far, the involvedness of JNK signalling has been mentioned various instances. Nevertheless, there is some controversy surrounding the function of JNK signalling in the Hippo pathway. A recent study described in ‘Sun G., and Irvine K.D., 201359’, shows its involvedness in regenerative growth. Regenerative growth requires the activation JNK signalling. This signalling route is activated under stress, for example by wounding, irradiation or oxidation. As a reaction to stress it can induce apoptosis and proliferation. Notwithstanding the controversy, both can be regulated via the Hippo pathway. JNK can be activated by Hpo to mediate apoptosis and activate Yki. In the case of regenerative growth, Yki gene transcription is up-regulated resulting in an increase in proliferation and a decrease in apoptosis. Epistasis experiments show JNK acts upstream of Wts to inhibit it from phosphorylating Yki. This inhibition requires the activity of dJub, a Drosophila orthologue of Ajuba LIM domain proteins59, 63. In Drosophila, loss of dJub phonocopies Yki deficiency. Co-immunoprecipitation shows dJub is able to interact with Sav and Wts, which is conserved as mammalian Ajuba proteins can interact with Lats and ww45. The interaction of dJub inhibits the phosophorylation of Yki 63. In vitro, the mammalian orthologue of JNK is shown to stimulate the binding of Ajuba proteins to Lats1 via phosphorylation. The binding of the Ajuba proteins prevent Lats1 to be phosphorylated by Mst59. Therefore, there is a connection between JNK and the Hippo pathway in the regulation of apoptosis and proliferation. Understanding of the exact mechanism of action requires further examination, though. 20 Taken together, there are many different regulators of the Hippo pathway. Furthermore, these regulators sometimes suggest connections to other mechanisms, such as polarity and metabolism. A schematic overview of the regulation is depicted in Figure 7. In order to look at Hippo signalling on a broader scale, the connections between cells in a tissue should be discussed. Therefore, cell-cell interactions influencing the Hippo pathway will be the main focus in the next chapter. Figure 7. Schematic overview of the upstream regulation of the Hippo pathway in Drosophila. Ex, Mer and Kibra positively regulate Hippo signalling upstream of Hpo and Sav. The precise mechanism remains elusive. Tao-1 phosphorylates and activates Hpo. dRASSF and dStripak negatively regulate Hippo signalling by dephosphorylating Hpo. The balance in polarity caused by mutual antagonism of aPKC and Lgl positively regulates the Hippo pathway. JNK signalling is required for the inhibition of Wts via the phosphorylation of dJub. There is a direct link between Hpo and JNK as well, however, surrounding this link lies a lot of controversy. Metabolism associated proteins like Akt and Sik-1/-2 negatively regulate the Hippo pathway via inhibition of Hpo and Sav respectively. Mechanical stress can influence Hippo signalling via a protein complex consisitng of Dachs, Zyx and App. The binding of Dachs to Wts inhibits its function possibly by facilitating the degradation of Wts. Note that some of the upstream Hippo pathway regulators are among the Yki targeted genes indicating a feedback mechanism. Many of the factors are conserved in mammals (Table 1, appendix), however, there are differences in regulation. For example, mammals have various RASSF homologues. RASSF1A is shown to positively regulate Mst1 (Hpo orthologue), whereas, RASSF1C is suggested to negatively regulate the Hippo pathway. 21 Chapter 4. Cell-cell interactions influence the regulation of the Hippo pathway The development and maintenance of tissues and organs is a tightly regulated and complex process. Many mechanisms are required to control size, shape and patterning. In order to coordinate this, communication between neighbouring cells and tissues is necessary. Therefore, a connection between the external environment and the internal signalling routes, to tightly control the regulation, is necessary. In the previous chapters a few inputs of the Hippo pathway were disclosed. Here, factors capable of transducing different input signals to regulate the Hippo pathway will be discussed. The transmembrane protein, Fat, mentioned briefly in chapter 3, is a factor capable of transducing external information. The gene was first described in a search for hyperplastic tumour suppressors in Drosophila64. Fat and Fat-like proteins are protocadherins that are part of the cadherin super family. Its distinctive extracellular domain, consisting of a large amount of cadherin repeats, sets these proteins apart from conventional cadherins. The proteins have a wide range of functions, i.e. functioning in cell-cell interactions, regulation of planar cell polarity (PCP), migration and growth control65, 66. Not surprisingly, its influence on the latter function, links the protein to Hippo signalling. Fat mutants in Drosophila phenocopy hpo mutants, thereby showing overgrowth and up-regulated diap1 and cycE. Several epistasis experiments placed it upstream of the Hippo pathway core kinases54, 67. Furthermore, co-immunoprecipitation indicated Fat negatively affects the phosphorylation of Wts. The link between Fat and Dachs, Zyxin and App was already explained in chapter 3. Fat inhibits the phosphorylation of Wts via inhibition of these proteins, thereby positively regulating the Hippo pathway54. The use of several cleaved fragments of Fat revealed that the intracellular domain of Fat is required for its influence on the Hippo pathway67. Actually, expression of the intracellular domain of Fat can rescue the fat mutant overgrowth phenotype, indicating Fat can compete for Dachs binding54. These results suggest Fat, with its extra- and intracellular domain, can function as a signal transducing receptor in the Hippo pathway. However, the fat mutant phenotypes are not as severe as complete loss of Hippo signalling, indicating it is not the only upstream regulator54, 67. The epistasis experiments performed showed Fat acts upstream of Ex as well, while functioning in parallel with Mer54, 67, 68. Fat revealed to be required for the localisation of Ex68. Upstream regulators of Fat and Fat-like cadherins were already revealed in a study focussing on planar cell polarity. Fat is able to interact with another protocadherin Dachsous (Ds). Furthermore, the Golgi protein Four-jointed (Fj) is involved69. Fat activity is negatively regulated upon Ds interaction. In fact, Ds regulates the phosphorylation of Fat via Disc overgrown/Casein Kinase 1δ/ε (DCO). Coimmunoprecipitation of several Fat deletion mutants show DCO is able to interact with the intracellular domain of Fat and phosphorylate several sites70. Fj negatively regulates Ds activity. In vitro and in cell culture, Fj was shown to be able to phosphorylate Fat and Ds, inhibiting the binding of the two protocadherins71. Additionally, the protein lowfat (Lft) is able to bind the intracellular domains of both Ds and Fat. Lft expression is responsible for elevated levels of Fat and Ds. Immunostaining shows Lft affects the protein levels, not the mRNA levels of Fat and Ds. These results suggest Lft regulates Fat 22 and Ds levels posttranscriptionally72. A schematic overview of Fat signalling in relation to Hippo signalling is depicted in Figure 10. An intriguing result arose when looking deeper into the regulation of Fat signalling. Both overexpression and depletion of Ds caused a down-regulation of Yki target genes73, 74. Figure 8 shows ds gain of function during Drosophila wing development causes growth defects. The down-regulation of the genes indicated induction of the Hippo pathway. However, it was controversial to see both a gain and a loss of function of ds causing similar effects. These results suggested it was not the absolute amount of the protein modulating the Hippo pathway. Moreover, when looking at mutant clones a clear pattern was visible. The Hippo pathway seemed repressed in the boundary of mutant clones rather than inside the mass. Apparently, the position of a cell on/near a clone boundary influenced the Hippo pathway. Subsequent experiments showed it was in fact the differences in Ds levels between neighbouring cells causing the effect. The same observations could be seen in fj mutant clones. Fj and Ds are expressed in a gradient71, 73, 74. At boundaries cells can interact with cells with different amount of Fj and/or Ds activity. Cells on both sides of the boundary can induce Yki target transcription in gradients up to several cells away from the boundary73, 74. Figure 8. Uniform expression of Ds and Fj inhibit growth in Drosophila adult wings. A tub-Gal4 transgene was used for ubiquitous expression. (A) Shows wildtype wing size, no UAS transgene was used. (B) Shows wings size when Ds is uniformely expressed. (C) Shows wing size when Fj is uniformely expressed. (D) Shows wing size when both Ds and Fj are uniformly expressed. Ds and Fj single mutants show an intermediate phenotype when compared to the more severe double mutant. The double mutant phenocopies Dachs depletion mutants, emphasising the link to Fat signalling. Results from Rogulja D., et al., 200873. The mechanism where Hippo signalling is controlled by differential levels of factors of juxtapositioning cells was called boundary signalling/the boundary effect (Figure 9). The existence of boundary signalling facilitates a link between PCP and growth control. PCP can cause gradient differences of proteins in a tissue. Differences of protein activity can subsequently modulate Fat and Hippo signalling. In fact, a known morphogen distributed in a gradient along the anterior-posterior axis in Drosophila wing development is involved in the expression gradient formation of Fj and Ds73. Another interesting implication of the boundary effect is the function of Ds. The extracellular domain of Ds can interact with Fat and modulate Fat signalling. For the generation of 23 the boundary signal, though, the expression of only the intracellular domain was sufficient. The intracellular domain was insufficient to render the cell capable of responding to the boundary. In order to generate the boundary effect and respond to the boundary signal both the intracellular and extracellular domain of Ds are required. The data suggest that, like Fat, Ds acts as a transducing receptor74. Even though, the specific mechanism of boundary signalling remains unknown, it is required for the modulation of the Hippo pathway and linking it to PCP. Figure 9. Schematic overview of the boundary effect. Cells in adjacent tissues have differing levels of Ds and Fj. At borders of tissue types, cells with the differing protein levels are juxtapositioned causing inhibition of the Hippo pathway. Note, that the boundary effect is explained here using proteins taking part in Fat signalling. The boundary effect was shown in Crb signalling as well. The involvement of Fat signalling in Hippo pathway modulation seems conserved in the vertebrate system. Genetically, the proteins are conserved. Furthermore, a homolog of Fat (Fat1) has similar results in Yap target regulation in Zebrafish (Danio rerio) 75. Interestingly, the homolog of Scr can interact with Fat1 and is required for the Fat1 regulation of Hippo signalling. Since, Scr is a polarity regulating protein, the link between polarity and Hippo signalling is emphasised in Fat1 signalling. The cross talk between different signalling cascades seems required to properly define pronephros size and structure in Zebrafish75. Another transmembrane protein involved in Hippo signalling is Crumbs (Crb). Crb is known to be involved in the regulation of apical basal cell polarity57, 76, 77. The protein localises at the apical cortex by self-recruitment, upon interaction between the extracellular domain of two Crb proteins in neighbouring cells78. The apical polarisation is antagonised by the polarity regulating proteins Dlg and Lgl, mentioned in chapter 3 77, 78. Mutations in crb phenocopy hpo mutants, with up-regulated Yki target transcription and induced proliferation. These results suggested Crb might function as an upstream regulator of the Hippo pathway76, 79. Epistasis experiments showed the transmembrane protein indeed functioned upstream of the Hippo pathway. Furthermore, Ex localisation seemed affected in crb mutants. Instead of localising apically, Ex distributed more diffusely upon crb mutation57, 76, 77. Co-immunoprecipitation coupled to cleavage experiments showed the intracellular domain of Crb was required for binding to and apical localisation of Ex76, 77. The domain required for Ex localisation differs from the polarisation regulation domain, though, indicating Crb can regulate growth and polarisation simultaneously77. Moreover, the apical recruitment of Ex, and Kibra as well, have been shown to stabilise Crb at the apical membrane in Drosophila follicle cells78. Normally, the antagonistic effects of Lgl induce endocytosis of Crb and subsequent loss of apical membrane identity. Recruitment of the Crb complex and the upstream Hippo signalling factors prevent this from happening78. Interestingly, follow up experimentation showed a loss of function mutation of crb causing the same phenotype as a gain of function mutation76, 79. These seemingly 24 contradictory results could be explained by the previously mentioned boundary effect73, 74, 79. Apoptosis seemed to be induced especially at clonal boundaries of mosaic animals. At these boundaries the levels of transmembrane Crb differed. Apparently, if Crb levels are similar, either high or low, in neighbouring cells, the Hippo pathway is downregulated and proliferation is induced. However, if Crb levels in adjacent cells differ, the Hippo pathway is induced and apoptosis is induced. These results suggest a non-cell autonomous function for Crb in tissue morphology. A cell can transduce a signal to induce apoptosis via the extracellular domain of Crb in a neighbouring cell79. This signal transduction could be used as a size sensing mechanism. As organs grow, mechanical forces could alter the geometric structure of an organ. This structure change can also lead to changes in Crb interactions. The competition between neighbouring cells and the added boundary effect will induce apoptosis and reduce organ size subsequently. Apoptosis is most often induced in both cells at the boundary. However, when boundary signalling and mutations in Hippo signalling are coupled, super competitive clones can consequently arise. Boundary signalling would induce apoptosis via crb in both wildtype and Hippo signalling mutant clones. Though, the Hippo pathway mutation induces Yki target transcription, such as diap1, making apoptosis evasion possible79. Therefore, instead of the induction of apoptosis in neighbouring cells at the boundary, the hpo mutant cells will be able to survive. In conclusion, Crb is an important transmembrane protein able to integrate signals from neighbouring cells to modulate the Hippo pathway accordingly. An additional transmembrane receptor involved in Hippo signalling was found in the human glioblastoma multiforme (BGM) cancer cell line. CD44 encodes a cell surface hyaluronan receptor. Depletion of the protein leads to sustained phosphorylation and activation of Mst1/2 and Lats1/2, resulting in inactivation of Yap. A reduced expression of cIAP1/2, human orthologues of diap1, was also observed14. High expression of the receptor gave the opposite effect, indicating the negative regulation function on the Hippo pathway in GBM cells. Overgrowth visible upon expression of CD44 was due to evasion of apoptosis mediated by the Hippo pathway via JNK signalling14. The connection of the CD44 receptor to the Hippo pathway did not come as a surprise since a link has been suggested previously. The cytoplasmic domain of CD44 has been shown to interact with Merlin. The binding of Merlin can inhibit the hyaluronan-CD44 interaction and thus prevent CD44 activity80. These results suggest CD44 is a negative regulator of the Hippo pathway. Recent studies have proposed a role for cytoskeleton integrity in modulating the Hippo pathway. Immunofluorescence assays show Mst can co-localise with Filamentous actin (F-actin) structures in mammalian cell lines 60. Furthermore, induction of extra F-actin polymerisation was shown to induce an overgrowth phenotype in Drosophila imaginal discs81. F-actin polymerisation was stimulated by depletion of capping proteins and expression an F-actin formation triggering protein (Diaphanous). Besides the overgrowth phenotype, F-actin formation leads to several Hippo pathway typical phenotypes, e.g. activation of Yki, up-regulation of diap1 and ex. These results show that changes in F-actin can modulate the Hippo pathway81. Evidence for a link between the Hippo pathway and the actin-cytoskeleton was then further emphasised in mammalian cell lines. Studies suggested the proteins Ajuba and Angiomotin (Amot) as possible links between the actin-cytoskeleton, F-actin and the Hippo pathway82, 83. 25 All in all, a connection between the actin-cytoskeleton and the Hippo pathway is clear. A possible functional explanation for this connection lies in the external to internal information transduction aspect. In Hela cells, the loss of cell-cell interactions led to the induction of F-actin polymerisation84. Apparently, the loss of information from neighbouring cells can be the cause for actin-cytoskeletal changes. As was mentioned previously, the F-actin changes leads to the disruption of the Hippo pathway and induction of Yki target genes necessary for proliferation and migration. This mechanism can be implicated in development. However, there is likely a role in wound healing as well. Cell interactions are often lost during wound formation. Stimulation of processes, such as proliferation and migration, can thus promote the healing process84. Taken together, these results indicate cell-cell interactions are required to transduce signals for the regulation of the Hippo pathway. Furthermore, the dual role of many factors in both polarity (PCP) and growth control is likely not a coincidence. Cross talk between both processes is likely necessary to achieve proper tissue and organ size, shape and patterning. The different intracellular signals involved in Hippo signalling are depicted in Figure 10. Figure 10. Schematic overview of intracellular interactions regulating the Hippo pathway. The apically localised transmembrane protein Crb can interact with Crb proteins from neighbouring cells. This interaction is required for its function in the localisation and consequent activation of Ex. Crb localisation and activity is affected by cell polarisation. The transmembrane protein Fat is localised apically as well. Binding with the transmembrane protein Ds of a neighbouring cell facilitates the phosphorylation of the intracellular domain of Fat by DCO. The intracellular domain of Fat is required for the inhibition of Dachs, an intracellular protein known to inhibit Hippo signalling. Protein levels of Fat and Ds are positively regulated by Lft. Both Crb signalling and Fat signalling, via Ds and Fj are regulated via the boundary effect, where differing protein levels in adjacent cells are required for Hippo pathway modulation. Another cell-cell interaction modulator of the Hippo pathway acts through adherens junctions (AJ). dJub negatively regulates Hippo signalling via these junctions. In humans dJub connects the adherens junctions via αcatenin to the actin-cytoskeleton via F-actin. This interaction connects the actin-cytokeleton to the Hippo pathway. However, this mechanism is not shown in Drosophila and is therefore 26 annotated by the asterisks. The fact that F-actin polymerization is able to modulate Hippo signalling in Drosophila indicates there might a similar mechanism, though. Most of the other regulators are conserved in mammals (Table 1, appendix). An additional transmembrane regulator in humans is CD44. This protein is shown to negatively regulate Hippo signalling in the glioblastoma multiforme cancer cell line. 27 Discussion Mechanisms involved in cell growth regulate normal development and homeostasis of shape, size and patterning of tissues/organs in multicellular organisms31, 85. One of the first mechanisms discovered involved in cell growth, by affecting both proliferation and apoptosis, is the Hippo pathway1, 3, 6, 8. Deregulation of this mechanism leads to severe effects involved in the development and progression of cancer. In the previous chapters many components of the Hippo pathway were shown deregulated in cancer cell lines1, 5, 6, 10, 12-15, 32. Furthermore, prolonged deregulation of the main effector, Yki, resulted in tumour formations in the liver of mice in vivo15. The severe impact of deregulated components on the balanced mechanism underscores the importance of tight regulation of the mechanism. In this review, the identification and the function of components of the Hippo pathway were reviewed. The core kinases, Hpo, Sav, Mats and Wts, of the pathway were first discovered in Drosophila melanogaster (chapter 1). Since the kinases negatively regulate growth they have been identified as tumour suppressors1, 3, 5, 6, 8, 26, 31. The Hippo pathway core kinases modulate the downstream activity of the oncogene yki via phosphorylation and subsequent inactivation of the encoding protein. Yki functions as a transcription co-activator, regulating the transcription of several target genes involved in proliferation and apoptosis evasion (chapter 2) 10. More recently, the function of the Hippo pathway has been ascribed a broader function than growth control alone. Several of the downstream targets have been implicated in processes such as cell cycle exit, differentiation and migration as well1, 4, 26, 86-88. In any case, Hippo signalling has been identified as an important mechanism for tissue/organ development and homeostasis. The importance of the core kinases and Yki is emphasized by the evolutionary conservation (Table 1, appendix) 2, 89, 90. Figure 11, shows these core components are conserved in Drosophila and humans. A recent publication, ‘Hilman D., and Gat U., 201390’, shows that conservation of the core kinases can even be deduced to organisms such as bakers yeast (S. cerevisae). The conservation of the key regulator, Yki, seems less apparent, though. For instance, whether the transcription co-activator is conserved in C. elegans, is still debated. Recent findings suggest the newly identified gene yap-1 as a homolog91, 92. Yap-1 partially shares the basal characteristics, sequence similarity in the N-terminal TEAD binding domain and WW domain, of mammalian Yap. Furthermore, knockdown of the C. elegans homologs of Wts and 14-3-3 show nuclear accumulation of Yap-191. These results suggest that Yap-1 is negatively regulated by Hippo pathway components, similarly to the regulation of Yki/Yap in other organisms. However, a regulative connection between the C. elegans homologs of Hpo and Yap-1 remains absent. It could be the connection is hard to show. However, it could also be that the Hippo pathway in C. elegans is conserved without Hpo itself taking part in the signalling route. This does not have to come as a surprise, since C. elegans is known as an organism with a fixed cell number and high invariance in development93. The already existing tight regulation of cell size, shape and patterning might thus make full activity of a Hippo pathway redundant. Bioinformatics might help to elucidate the evolutionary conservation of the Hippo pathway further. 28 Figure 11. Evolutionary conservation of the Hippo pathway core components. The schematic overview shows the genetic and functional conservation of the core components of the Hippo pathway. The most important downstream target is conserved as well. Note the duplication of Mst1/2 (Hpo), Lats1/2(Wts), Mob1A/B(Mats) and Yap/Taz (Yki). While the conservation of the core components of the Hippo pathway seems relatively clear, the conservation of the upstream regulators is less apparent. The upstream regulation of Hippo signalling is rather extensive (chapter 3) 45. Many factors are involved, amongst which Ex, Mer and Kibra are probably the best known12, 46-48. The extensiveness of the regulation makes many of the components at least partially redundant between many tissues and organisms. An example is the tissue and timing dependent requirement for Ex and Mer in Drosophila, where Mer is more required in posterior follicle cells in the oocyte and Ex more in eye imaginal discs during larval development47, 48. Furthermore, there seem to be differences in the conservation of many factors between organisms89, 90, 92. For instance, several factors, within the core pathway as well, have more than one homolog in more complex organisms89, 90. Figure 11 shows situations where Drosophila has only one copy of a certain gene and humans have more. Duplication followed by diversification is a process commonly observed when looking at the evolution of a mechanism. The more complex an organism becomes the more complex signalling routes are required. The rise in complexity can be due to an increase in timing and/or tissue specificity, but also due to increased cross talk between mechanisms and/or tissues. The integrator model mentioned in ‘Yu J., et al., 201048’ is in concordance with this model of evolution. The core kinases may act as signal integrators and are well conserved and ubiquitously required in most organisms. The upstream signals come from different regulators, which are less conserved and required in a timing or tissue specific manner. However, note that some of the Hippo pathway factors have been shown to modulate cell growth independently from the core kinases as well12, 47, 48. Ex is probably the most explanatory example, since it appears to negatively regulate Yki activity independently of the core kinases12. Furthermore, other signalling routes, such as Wnt and GPCR, have been shown able to regulate Yki/Yap 29 activity94. Therefore, even though the core of the Hippo pathway is important and conserved, it is only a small piece of the puzzle. Aside from the internal regulators, many external signals are integrated to modulate Hippo signalling. Growth and other processes can be regulated in one cell on its own. However, for proper development and homeostasis of size, shape and patterning of tissues, various inputs from the exterior are required (chapter 4). Many transmembrane proteins are involved in receiving external signals and transducing them interiorly to the Hippo pathway. Among these proteins are Fat and Crb66, 67, 76, 77, 95, 96. Ligand binding to the extracellular domain of either proteins results in Hippo pathway activation. This is a mechanism through which overall tissue size and shape can be controlled. When the shape of a tissue/organ increases the density of cells increases as well. The increase in density or ‘crowding’ is thought to facilitate cell-cell interactions. Subsequent to the increase in interactions, the Hippo pathway is activated to prevent further proliferation and/or induce apoptosis79. These specific reactions counter the ‘crowding’ effect and overall size is maintained/reduced. In other words, contact inhibition takes place to modulate tissue/organ size. Vice versa, when tissue size is smaller the lack of interactions causes reduced Hippo signalling activity, resulting thus in overall tissue growth. However, in both Fat and Crb signalling the boundary effect plays a role as well. Differing protein levels in adjacent tissues are required for proper Hippo signalling modulation73, 74. Not surprisingly, polarization is required for proper functioning of these transmembrane proteins57, 74, 76-79, 82. When active, the transmembrane proteins are mostly localized at the apical membrane, where many of the Hippo pathway components localize as well. Proper localization of many of the Hippo pathway components is actually dependent on proper localization of upstream regulators56-58, 77, 82, 97. The loss of polarity might thus indirectly affect the Hippo pathway by mislocalization of several apically required factors. Furthermore, the above mentioned boundary effect requires polarization. Note that, at least in the case of Fat signalling, gradient formation of the ligand is required. The formation of this gradient is dependent on a morphogen gradient affecting the patterning of Drosophila wing during development73. This example illustrates how not only polarity within a cell but also patterning within a tissue is required for proper Hippo regulation. A clue that polarity requirement in Hippo signalling might be more intricate arises when looking at migration. Tumour progression is often accompanied by invasion. In order for invasion to take place certain polarity needs to be lost16. Or, in other words, contact inhibition between neighbouring cells needs to be lost. Experiments done in human ovarian cancer cell lines showed the loss of contact inhibition was promoted by overexpression of Yap2. Furthermore, the overexpression induces migration subsequently98. In Drosophila border cell migration regulation of the Hippo pathway was shown to be involved as well16. Apparently, Wts is able to phosphorylate and inhibit ENA, an actin regulator required for migration speed. Within the cluster of cells, cell-cell interactions are affluent and Hippo signalling is activated. Therefore, cells inside the cluster will not readily migrate because of ENA inhibition. Cells on the border of the cluster, though, lack many of the cell-cell interactions. The lack of Hippo signalling makes the cells in the outer rim capable of migration. The migration of the border cells is thought to ‘pull’ the rest of the cluster ‘along’16. Both these studies suggest the interactions between cells in a tissue are required for proper Hippo regulation. Cell-cell interactions are regulated in part by polarisation, making it thus an important Hippo pathway modulator. Once polarity is lost cells are no longer bound to their position and migration/invasion is 30 facilitated. The regulation of migration of course can be important in processes such as wound healing. However, as mentioned previously, when deregulated it is an important factor involved in tumour progression. When addressing the topic of tissue organisation, the influence of mechanical stress and the involvement of the actin-cytoskeleton need to be mentioned. Cell-cell interactions are not only elicited by transmembrane proteins, such as Fat and Crb, connections can be made via adherens junctions also81, 84. The actin-cytoskeleton of neighbouring cells is connected via these adherens junctions. Mechanical stress is known to influence the (de)polymerization of the actin-cytoskeleton in cells55, 81, 84. The stress signals can be transduced via the adherens junctions. Transduction of these signals makes it thus possible to facilitate cytoskeletal changes throughout a tissue. Moreover, F-actin changes can modulate the Hippo pathway81. There is a lot of cross talk visible between the cytoskeleton conformation and the Hippo pathway55, 83, 84, 99, 100. However, how one mechanism/process regulates the other precisely and vice versa is still relatively unknown. Taken together, the connections between cells are again required for the organisation, size and shape of tissues via the Hippo pathway. Another factor, which might be involved in the modulation of the Hippo pathway, is nutrient availability. If the Hippo pathway is indeed involved in integrating external signals for proper tissue development and homeostasis, this does not come as a surprise. Controlling growth to situations where nutrients are available seems advantageous. Indeed, proteins such as Akt and Sik, which are involved in metabolism, have connections to the Hippo pathway13, 22. Furthermore, a recent study shows Hpo is able to modulate fat storage through adipocyte proliferation in Drosophila101. The diverse and extensive regulation via several input signals and upstream regulators are not the only factors making the Hippo pathway a complex mechanism. The Hippo signalling pathway does not just act as an on/off switch. There are several feedback loops involved to coordinate a strict balance50. As mentioned in chapter 3, Yki activation targets the transcription of several upstream regulators of the Hippo pathway, such as Ex, Mer and Kibra12, 46-48. Here, transcription negatively regulates its own activity. Hippo activity also affects the abovementioned processes such as polarity and cytoskeletal conformation, thus, indirectly affecting the Hippo pathway itself as well84, 94. Note that the changes can also affect neighbouring cells; the feedback loops have more than just a cell autonomous effect. Taken together, the Hippo pathway is a complex and highly regulated mechanism involved in growth control. A schematic overview of the various input signals and downstream effects is illustrated in Figure 12. Regulation of the pathway differs between tissues and organisms. Furthermore, there is feedback and cross talk with many other mechanisms. Conservation of many of the core components shows the mechanism is of great importance. The importance is further emphasised by the severe tumorigenic effects caused when the signalling route is deregulated. Therefore, extensive research to further elucidate the different mechanisms and involved regulators to fully understand its function in relation to tissue development and homeostasis is essential. 31 Figure 12. Schematic overview of the various input signals and downstream effects of the Hippo pathway. In tissues/organs various signals can affect the Hippo pathway core kinases (Hippo core) to facilitate downstream regulation. Polarity, dynamics of the actin-cytoskeleton, cell-cell interactions and metabolism are among these input signals. However, there might be other signals, which have not been discovered. The downstream target of the Hippo core is the transcription co-activator Yki. Here, the co-activated transcription factor is indicated as Tcf. Yki can be regulated directly by some regulators of the Hippo core and possibly other factors as well. Targets of Yki-transcription factor signalling are genes involved in processes such as proliferation, apoptosis, cell-cycle exit, differentiation, polarity migration, metabolism and Hippo pathway regulators. The input signals and downstream effects can be tissue, timing and/or organism specific. 32 Appendix Components of the Hippo pathway in Drosophila and mice (mammals). Conserved protein functions are mentioned in the third column. From Halder G., and Johnson R.L., 2006 2. *The mammalian Mst1 and Mst 2 kinases require cleavage prior to activation. Drosophila does require similar cleavage3, 4, 26-28. IMammalian Yap and Taz contain a PDZ binding motif, required for nuclear localization and proapoptotic signalling, which is not present in Drosophila Yki. Yap and Taz are phosphorylated by Lats and a different kinase as well. Phosphorylation mediates the ubiquitination of Yap and Taz and label the proteins for subsequent degradation. ±Drosophila does not have a CD44 homolog. §There is no direct homolog of Dachs known in vertebrates. ¶The sequence of the mammalian Ex homologs diverge from Drosophila Ex12. 33 ReferencesReferences 1. Tapon, N. et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467-478 (2002). 2. Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9-22 (2011). 3. Wu, S., Huang, J., Dong, J. & Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445-456 (2003). 4. Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. & Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914-920 (2003). 5. Rothenberg, M. E. & Jan, Y. N. salvador--The persistence of proliferation. Cancer. Cell. 2, 171173 (2002). 6. Kango-Singh, M. et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719-5730 (2002). 7. Lai, Z. C. et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120, 675-685 (2005). 8. Justice, R. W., Zilian, O., Woods, D. F., Noll, M. & Bryant, P. J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534-546 (1995). 9. Xu, T., Wang, W., Zhang, S., Stewart, R. A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053-1063 (1995). 10. Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421-434 (2005). 11. Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747-2761 (2007). 12. Hamaratoglu, F. et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8, 27-36 (2006). 13. Zhang, L. et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell. 14, 377-387 (2008). 14. Xu, Y., Stamenkovic, I. & Yu, Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 70, 2455-2464 (2010). 15. Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120-1133 (2007). 16. Lucas, E. P. et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 201, 875-885 (2013). 34 17. Zhang, H., Pasolli, H. A. & Fuchs, E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. U. S. A. 108, 22702275 (2011). 18. Tyler, D. M. & Baker, N. E. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev. Biol. 305, 187-201 (2007). 19. Hanahan, D. & Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell 144, 646-674 (2011). 20. Ye, X., Deng, Y. & Lai, Z. C. Akt is negatively regulated by Hippo signaling for growth inhibition in Drosophila. Dev. Biol. 369, 115-123 (2012). 21. Yuan, Z. et al. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J. Biol. Chem. 285, 3815-3824 (2010). 22. Wehr, M. C. et al. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat. Cell Biol. 15, 61-71 (2013). 23. Tao, W. et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat. Genet. 21, 177-181 (1999). 24. Knoblich, J. A. et al. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77, 107-120 (1994). 25. Hawkins, C. J., Wang, S. L. & Hay, B. A. A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP1. Proc. Natl. Acad. Sci. U. S. A. 96, 2885-2890 (1999). 26. Pantalacci, S., Tapon, N. & Leopold, P. The Salvador partner Hippo promotes apoptosis and cellcycle exit in Drosophila. Nat. Cell Biol. 5, 921-927 (2003). 27. Jia, J., Zhang, W., Wang, B., Trinko, R. & Jiang, J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514-2519 (2003). 28. Harvey, K. F., Pfleger, C. M. & Hariharan, I. K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457-467 (2003). 29. Chan, E. H. et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076-2086 (2005). 30. Praskova, M., Xia, F. & Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311-321 (2008). 31. Wei, X., Shimizu, T. & Lai, Z. C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 26, 1772-1781 (2007). 32. Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747-2761 (2007). 33. Oh, H. & Irvine, K. D. In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081-1088 (2008). 35 34. Vassilev, A., Kaneko, K. J., Shu, H., Zhao, Y. & DePamphilis, M. L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229-1241 (2001). 35. Badouel, C. et al. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev. Cell. 16, 411-420 (2009). 36. Oh, H. & Irvine, K. D. In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916-1927 (2009). 37. Ren, F., Zhang, L. & Jiang, J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev. Biol. 337, 303-312 (2010). 38. Oh, H., Reddy, B. V. & Irvine, K. D. Phosphorylation-independent repression of Yorkie in FatHippo signaling. Dev. Biol. 335, 188-197 (2009). 39. Ziosi, M. et al. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 6, e1001140 (2010). 40. Nolo, R., Morrison, C. M., Tao, C., Zhang, X. & Halder, G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr. Biol. 16, 1895-1904 (2006). 41. Peng, H. W., Slattery, M. & Mann, R. S. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 23, 2307-2319 (2009). 42. Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962-1971 (2008). 43. Wu, S., Liu, Y., Zheng, Y., Dong, J. & Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell. 14, 388-398 (2008). 44. Goulev, Y. et al. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumorsuppressor pathway in Drosophila. Curr. Biol. 18, 435-441 (2008). 45. Kwon, Y. et al. The Hippo signaling pathway interactome. Science 342, 737-740 (2013). 46. Baumgartner, R., Poernbacher, I., Buser, N., Hafen, E. & Stocker, H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell. 18, 309-316 (2010). 47. Genevet, A., Wehr, M. C., Brain, R., Thompson, B. J. & Tapon, N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell. 18, 300-308 (2010). 48. Yu, J. et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell. 18, 288-299 (2010). 49. Yang, S. et al. Phosphorylation of KIBRA by the extracellular signal-regulated kinase (ERK)ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration. Cell. Signal. 26, 343351 (2014). 50. Polesello, C., Huelsmann, S., Brown, N. H. & Tapon, N. The Drosophila RASSF homolog antagonizes the hippo pathway. Curr. Biol. 16, 2459-2465 (2006). 36 51. Oh, H. J. et al. Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res. 66, 2562-2569 (2006). 52. Tommasi, S. et al. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 65, 92-98 (2005). 53. Ribeiro, P. S. et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521-534 (2010). 54. Cho, E. et al. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38, 1142-1150 (2006). 55. Rauskolb, C., Pan, G., Reddy, B. V., Oh, H. & Irvine, K. D. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 9, e1000624 (2011). 56. Verghese, S., Waghmare, I., Kwon, H., Hanes, K. & Kango-Singh, M. Scribble acts in the Drosophila fat-hippo pathway to regulate warts activity. PLoS One 7, e47173 (2012). 57. Grzeschik, N. A., Parsons, L. M., Allott, M. L., Harvey, K. F. & Richardson, H. E. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573-581 (2010). 58. Grzeschik, N. A., Parsons, L. M. & Richardson, H. E. Lgl, the SWH pathway and tumorigenesis: It's a matter of context & competition! Cell. Cycle 9, 3202-3212 (2010). 59. Sun, G. & Irvine, K. D. Ajuba family proteins link JNK to Hippo signaling. Sci. Signal. 6, ra81 (2013). 60. Densham, R. M. et al. MST kinases monitor actin cytoskeletal integrity and signal via c-Jun Nterminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol. Cell. Biol. 29, 63806390 (2009). 61. Poon, C. L., Lin, J. I., Zhang, X. & Harvey, K. F. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev. Cell. 21, 896-906 (2011). 62. Boggiano, J. C., Vanderzalm, P. J. & Fehon, R. G. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell. 21, 888-895 (2011). 63. Das Thakur, M. et al. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 20, 657-662 (2010). 64. Watson, K. L., Justice, R. W. & Bryant, P. J. Drosophila in cancer research: the first fifty tumor suppressor genes. J. Cell Sci. Suppl. 18, 19-33 (1994). 65. Angst, B. D., Marcozzi, C. & Magee, A. I. The cadherin superfamily: diversity in form and function. J. Cell. Sci. 114, 629-641 (2001). 66. Mahoney, P. A. et al. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 67, 853-868 (1991). 67. Willecke, M. et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16, 2090-2100 (2006). 68. Feng, Y. & Irvine, K. D. Fat and expanded act in parallel to regulate growth through warts. Proc. Natl. Acad. Sci. U. S. A. 104, 20362-20367 (2007). 37 69. Yang, C. H., Axelrod, J. D. & Simon, M. A. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108, 675-688 (2002). 70. Sopko, R. et al. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr. Biol. 19, 1112-1117 (2009). 71. Brittle, A. L., Repiso, A., Casal, J., Lawrence, P. A. & Strutt, D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr. Biol. 20, 803-810 (2010). 72. Mao, Y., Kucuk, B. & Irvine, K. D. Drosophila lowfat, a novel modulator of Fat signaling. Development 136, 3223-3233 (2009). 73. Rogulja, D., Rauskolb, C. & Irvine, K. D. Morphogen control of wing growth through the Fat signaling pathway. Dev. Cell. 15, 309-321 (2008). 74. Willecke, M., Hamaratoglu, F., Sansores-Garcia, L., Tao, C. & Halder, G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 105, 14897-14902 (2008). 75. Skouloudaki, K. et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc. Natl. Acad. Sci. U. S. A. 106, 8579-8584 (2009). 76. Ling, C. et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. U. S. A. 107, 10532-10537 (2010). 77. Robinson, B. S., Huang, J., Hong, Y. & Moberg, K. H. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20, 582-590 (2010). 78. Fletcher, G. C., Lucas, E. P., Brain, R., Tournier, A. & Thompson, B. J. Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr. Biol. 22, 1116-1122 (2012). 79. Hafezi, Y., Bosch, J. A. & Hariharan, I. K. Differences in levels of the transmembrane protein Crumbs can influence cell survival at clonal boundaries. Dev. Biol. 368, 358-369 (2012). 80. Bai, Y. et al. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumorsuppressor activity of merlin. Oncogene 26, 836-850 (2007). 81. Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325-2335 (2011). 82. Hirate, Y. et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 23, 1181-1194 (2013). 83. Marie, H. et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J. Biol. Chem. 278, 1220-1228 (2003). 84. Reddy, P., Deguchi, M., Cheng, Y. & Hsueh, A. J. Actin cytoskeleton regulates Hippo signaling. PLoS One 8, e73763 (2013). 85. Potter, C. J. & Xu, T. Mechanisms of size control. Curr. Opin. Genet. Dev. 11, 279-286 (2001). 38 86. Lucas, E. P. et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 201, 875-885 (2013). 87. Dai, X. et al. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J. Biol. Chem. 288, 34041-34051 (2013). 88. Hwang, J. & Pallas, D. C. STRIPAK complexes: Structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 47, 118-148 (2014). 89. Bossuyt, W. et al. An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene (2013). 90. Hilman, D. & Gat, U. The evolutionary history of YAP and the hippo/YAP pathway. Mol. Biol. Evol. 28, 2403-2417 (2011). 91. Iwasa, H. et al. Yes-associated protein homolog, YAP-1, is involved in the thermotolerance and aging in the nematode Caenorhabditis elegans. Exp. Cell Res. 319, 931-945 (2013). 92. Yang, Z. & Hata, Y. What is the Hippo pathway? Is the Hippo pathway conserved in Caenorhabditis elegans? J. Biochem. 154, 207-209 (2013). 93. Sulston, J. E. & Horvitz, H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56, 110-156 (1977). 94. Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047-1059 (2013). 95. Chen, C. L. et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 107, 15810-15815 (2010). 96. Silva, E., Tsatskis, Y., Gardano, L., Tapon, N. & McNeill, H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr. Biol. 16, 20812089 (2006). 97. Matakatsu, H. & Blair, S. S. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr. Biol. 18, 1390-1395 (2008). 98. Hall, C. A. et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 70, 8517-8525 (2010). 99. Sansores-Garcia, L. et al. Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr. Biol. 23, 229-235 (2013). 100. Langer, E. M. et al. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev. Cell. 14, 424-436 (2008). 101. Huang, H., Wu, W., Zhang, L. & Liu, X. Y. Drosophila ste-20 family protein kinase, hippo, modulates fat cell proliferation. PLoS One 8, e61740 (2013). 39 40