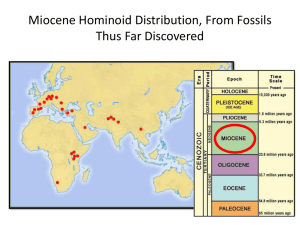
Versie 3 word 2009
advertisement

Evolutionary adaptation in the Spanish Anchitheriinae in relation to environmental and climatic changes. Masterthesis Biogeology Stella Heijnens 3021394 Supervisors; Dr. W. Wessels Prof. Dr. J.W.F. Reumer 1 Index - General introduction 3 - Thesis introduction 4 Main questions, outline and problems Chapter 1 - Background and Setting 7 - European Miocene Climate 7 - European Miocene Environment 8 - Anchitherium – MN3-MN9 9 o o - Taxonomy Species included Hipparion – MN9 o o 10 Taxonomy Species included - Systematic Paleontology Anchitherium 12 - Distribution 16 - Geological setting 16 - Biochronology 19 Chapter 2 – Literature Research and Methods - Evolution and adaptation o o o 22 22 Body and tooth size Micro and Mesowear Isotope analysis - Hypsodonty 25 - Climate and environment 26 o o Palaeoclimate and proxies Palaeoenvironment and proxies Chapter 3 - Practical research 31 Chapter 4 – Discussion 55 Chapter 5 – Conclusion 59 - References 62 2 Introduction The Anchitheriinae are an extinct subfamily of Equidae that first appeared in North America near the base of the Miocene. The Anchitheriinae clade migrated via Asia to Europe and has its first occurrences in Europe during the middle Orleanian, MN 3. The entire genus became extinct in the Vallesian, MN 9. Thus the genus existed in Europe approximately from 20 until 10 Ma ago (Kaiser 2009, Forsten 1991) (for MN zonation see de Bruijn 1992). Anchitheriinae have generally been considered to be browsing horses that occupied forested habitats because of their low crowned, lophodont teeth (Forsten 1991, Janis 1995). One of the classic examples of faunal turnover in the fossil record is the Late Miocene transition from faunas dominated by Anchitheriinae with low crowned molar teeth to faunas with Hipparion horses characterized by high-crowned teeth (Eronen et al. 2009, Strömberg 2005). This spread of Hipparion horses is often associated with the expansion of open habitats and also the increase of C4 grasses. Both in the New and the Old World fossil material of horses consists mainly of isolated teeth (Forsten 1991). It is generally accepted that Anchitheriine horses did not display an evolutionary increase in tooth crown height prior to their extinction (Eronen et al. 2009). “Lineages that remained as browsers in the Mid Miocene did not later transform into grazers, the general evolutionary pattern in the late Miocene is for browsing lineages to be replaced by ones adapted to more fibrous diets rather than the browsers themselves undergoing evolutionary change” (Janis 1994). Recent research on Anchitherium teeth as well as body size and isotopic composition of their dentition has shown however that adaptations to the overall changing environments in Europe as well as to a changing local climate and vegetation may have occurred in the Anchitheriine genus after all (Forsten 1991, Eronen et al. 2009, Kaiser 2009). Increased crown height has traditionally been used as an indicator for a change in diet towards more abrasive foods, often towards eating (C4) grass (Retallack 1983, Janis 1995, Fortelius et al. 2002). Therefore it has also been used as an indicator for environmental change, shifts towards a more open vegetation and as an aridity proxy (Janis 1995, Fortelius et al. 2002). An increase in abrasiveness of the food may not necessarily be due to phytoliths in grass, it may also be caused by increased fibrousness, extraneous dust or a decrease in nutritive value that requires the animal to increase its food uptake (Fortelius et al. 2002, Strömberg 2005). There is also discussion about whether hypsodonty is a direct evolutionary adaption or whether it might be due to phylogenetic effects, or ‘exaptation’ (Gould and Vbra 1981). Exaptation is a term coined by Gould and Vbra for traits shaped by selection to perform a different function than they are presently serving, or that result from other processes than natural selection. Therefore using it as a proxy for environment or climate may be questionable. 3 Thesis introduction, Anchitherium Anchitherium (meaning near-beast) is a small three toed horse that originates from the genus Miohippus in North America during the Early Miocene. As Anchitherium moved through Asia into Europe (MN 3) it underwent a phase of major diversification, among others giving rise to the much larger genus Sinohippus. Anchitherium is part of the first of four equid migrations from North America to the Old world. The second migration wave brought the genus Hipparion to the Old world and after a short period of co-occurrence the genus Anchitherium went extinct during the Late Miocene (MN 9) (Salesa et al. 2004). Anchitherium is generally thought to be a browsing type of horse living in forested environments because of its three toes and low crowned, lophodont teeth (Forsten 1991, Janis 1995). Classically the extinction of the genus Anchitherium has been seen as a failure to adapt to the more open and more arid vegetation of the Late Miocene. The Hipparion horse with its high-crowned teeth is generally seen as the genus outcompeting the low-crowned Anchitherium in a changing environment where Hipparion has the evolutionary advantage (Eronen et al. 2009, Strömberg 2005). Recently however Eronen et al. (2009) and Kaiser (2009) presented research showing that Anchitherium may yet have adapted to the dryer conditions of the early Miocene. Eronen et al. (2009) propose that Anchitheriines in Spain show ‘incipient hypsodonty’ and argue that this is evidence that they did respond to the increasing aridity of Europe that started in the Iberian region. They compare Spanish specimens with specimens found in Germany and show by means of cluster analysis of fossil Anchitheriinae and modern day ungulate tooth morphology that Spanish specimens cluster with ungulates that eat foliage and grass and the German specimens cluster with modern day ungulates that eat mostly foliage. They conclude that the Spanish species were slightly more adapted to drier conditions than the German species and that Anchitherium may not be the purely browsing horse that it was always thought to be. A problem concerning Eronen who links Anchitherium’s ‘incipient hypsodonty’ to a diet of more abrasive food and an adaptation to drier conditions is brought up by Kaiser (2009). According to Kaiser 2009 ‘there is a growing consensus that many species of Neogene equids showing increased crown height were in fact mixed feeders and even browsers’ (Hayek et al. 1991, Quade et al. 1994, MacFadden et al. 1999, Kaiser et al. 2000, Kaiser 2003). This doesn’t rule out the possibility Eronen suggests, that Anchitherium may have become a mixed feeder while adapting to dryer conditions. But it does indicate that hypsodonty is not necessarily a good indicator of adaptation to eating dryer, more grass like vegetation. However, Kaiser (2009) also concludes that Anchitherium might yet have adapted to a mixed feeding strategy. He bases his results on a different proxy, the mesowear patterns in the fossil teeth of Anchitherium. This proxy was developed by Fortelius and Solounias in 2000. He concludes that these horses may have had a more abrasive diet than previously thought. Kaiser concludes that members of the ‘Cormohipparion clade’ or Hipparion horses, that arrived in Europe from North America, introduced competition for Anchitherium in its feeding niche. He proposes that this was the case because Anchitherium had already 4 developed a mixed feeding strategy and Hipparion was also eating grass. Unfortunately Kaiser (2009) does not explain the reason why Anchitherium had turned this strategy, whereas Eronen et al. (2009) point to climatic and environmental change especially in Spain. An interesting point raised by Kaiser and Solounias (2003) is that Hipparion upon entering Europe changed its feeding strategy back to a more intermediate diet and even browsing forms established in Central Europe. This then would be in support of a hypothesis that Anchitherium may have changed to mixed feeding as an answer to competition in its dietary niche. Salesa et al. (2010) comment on Eronen et al. (2009) with two very valid points of critique. First of all they point out that Eronen et al. (2009) do not take into account the actual diversity of the Iberian representatives of Anchitherium. Therefore a comparison between the specimens from Germany, which are very homogeneous because they come from a single locality, and specimens from Spain, which represent a wide spatial range, is not possible. This taxonomic comment of Salesa et al. (2010) on Eronen’s work is very important and not only for Eronen’s research but for this thesis as well. The second point of critique is that Eronen et al. (2009) not only use Spanish fossils from a wide spatial range but from a wide temporal range as well. The German specimens in contrast are all from the same time period. The Spanish fossils are from many different fossil sites and all represent different time periods. According to Salesa et al. (2010) the comparison between Spain and Germany as a whole is invalid based on these errors. Eronen et al. (2010) subsequently reply pleading for the usefulness of looking at adaptations on the genus level. Main questions and complicating factors The interesting point brought up by Eronen et al. (2009) and also by Kaiser (2009) remains and gives rise to more questions that form the main motivation for writing this thesis; - Did Anchitherium adapt to eating grass (mixed feeding) or did Anchitherium fail to adapt to the changing environmental conditions of the Early Late Miocene? - Shortly after the Hipparion-date, did Anchitherium go extinct because it didn’t adapt or didn’t adapt quick enough to a changing environment or was it due to competition of the Hipparion horse in its feeding (Kaiser 2009) and or living niche? - Did the environment change so drastically during the early Late to Late Miocene that Anchitherium was likely to be forced into evolutionary adaptation or face extinction? Apart from the supposed evolutionary changes that occurred within Anchitherium there are many complicating factors that make this a very non-straight forward problem. Most of these factors will be discussed in following separate chapters, they will be shortly introduced here; Incipient hypsodonty, as Eronen proposes the Spanish Anchitheriinae had developed, may not necessarily be an evolutionary adaptation specifically for eating grass or even for excessive tooth wear. A discussion about the development of hypsodonty is still ongoing. 5 And even if hypsodonty is an adaptation for excessive wear, it may be caused by other things apart from eating grass such as; grid content, extrageneous dust or increased food uptake due to switching to a less nutritious food source (Strömberg 2005, Eronen et al. 2009). Simply looking at the level of hypsodonty will therefore not be enough to shed light on the main questions of this thesis. Furthermore Anchitherium went extinct shortly after the ‘Hipparion-date’ (Kaiser 2009, Forsten 1991). This may indicate that the reason Anchitherium went extinct was due to competition of Hipparion. Classically Anchitherium is seen as a browsing horse and Hipparion as one of the first hypsodont grazing/mixed feeding horses. So within the feeding niche these two genera should not present a threat to one another. If they did however invade each other’s feeding niche, there are technically three possible hypotheses; - Hipparion changed its feeding strategy to mixed feeding or browsing (Kaiser and Solounias 2003) thus invading Anchitherium’s browsing niche. - Anchitherium changed its feeding strategy towards more mixed feeding and therefore partly entered the in-migrating Hipparion’s grazing niche. - The last possible hypothesis is that both horses changed their feeding strategies towards mixed feeding and thus occupied the same feeding niche. The evidence for a shift towards more mixed feeding by Anchitherium that is presented by Kaiser (2009) and Eronen et al. (2009) suggests that this shift happened before the Hipparion-date. And Kaiser and Solounias (2003) suggest that Hipparion also changed its feeding strategy from grazing to a more mixed feeding to even browsing strategy upon entering Europe. This would make sense because changing to a feeding niche that is already occupied by another species is not a logical evolutionary step. So whichever adaptation took place, by Anchitherium, by Hipparion or by both, it most likely happened before Hipparion entered Anchitheriine territory. Competition in a spatial sense is also a form of competition as it is generally uncommon for two equine genera or even two equine species to inhabit the same habitat. We see this in fossil as well as in modern living assemblages (Janis, 2008). This however may also be closely linked to equine species often sharing the same food source. Also if Anchitherium were a pure browsing horse it would live in the forest and the grazing Hipparion would inhabit the plains, which were present at the time. So basically the spatial competition for the most part comes down to the competition for the feeding niches. The role of climate and environment in this discussion is also not at all straightforward. According to Eronen et al. (2009) the evolutionary change in Anchitherium is brought on by climatic and environmental change. The Miocene aridification that supposedly started on the Iberian Peninsula may have forced Anchitherium to start drawing upon other food sources besides leaves. But was the Spanish climate really overall dryer? Many researchers agree that the Miocene climate became dryer and cooler but many also point to the large regional differences on the European continent and also especially on the Iberian Peninsula. Another ongoing discussion deals with the spread of the grasslands. Did environmental 6 conditions really change so drastically from woodland to open savannah that Anchitherium had to adapt in order to survive? General practical problems During the course of the literature research part of this thesis some problems had to be solved or evaded. The various technical problems faced during the practical part will be discussed separately in part 3 of the thesis. A main and ever returning challenge of the literature part of this thesis is the diversity of languages in which the research about the genus Anchitherium is presented. For this thesis it was chosen to ignore all articles published in languages like Russian or Chinese. A couple of articles written in Spanish (Sánchez et al. 1998, Hernández Fernández et al. 2003) and in French (Alberdi et al. 2004) were used. Another problem was the fact that some authors presented incomplete and in some cases even incorrect information. Taxonomic references were incomplete or missing and materials and methods were often unclear. Terminology and nomenclature was also used in different ways by different authors and in different languages. Sometimes figures were even printed upside down switching lingual and labial side. In this thesis it has been attempted to add as much information about data, terminology, taxonomy and methodology as possible. Chapter 1 - Background and setting This part of the thesis will consist of a general introduction to Miocene climate and environment. What are the major geological events influencing Miocene climate? In part 2 climate and environment will be discussed to much greater detail using many different researches and proxies. Part 1 will form the background for the rest of the thesis. The background of the genera Anchitherium and Hipparion will also be presented. Their taxonomy and which species of the genera are included in the research. The distribution of the different genera and species, the geological setting of the fossil finds and finally the general biochronology will be discussed in this chapter. The geological setting of the fossil finds that were used in the practical research will be discussed in part 3. European Miocene climate The Neogene climate system represents the transition from the greenhouse climate of the Paleogene to the icehouse climate of the Quarternary. Within the Neogene the Miocene is considered the most critical interval in the build-up of ice masses on land (Bruch et al. 2007). Severe climatic and environmental changes occurred especially during the late Miocene, the uplift of the Tibetan Plateau was an important geological cause for these changes, it influenced for example the East Asian monsoon. Another global factor that may have driven a major climatic change is the closure of the Panama Ishtmus. This event took place between 13 and 2.6 Ma and resulted in a decrease in the mixing of Atlantic and Pacific waters which gave rise to the development of the modern Atlantic thermohaline circulation (Domingo et al. 2008). During the early Miocene, an overall trend of increasing temperature is observed by Mosbrugger et al. (2005), the curve however shows short term variations, These which will be discussed further in Part two: climatic reconstructions and proxies. A short term 7 cooling is observed at the base of the Aquitanian (MN1 - MN2), in the later Burdigalian (MN3 - MN4) temperatures increase again and the succeeded into a warm time span that persisted through the earlier part of the Serravallian (MN7/8). This warm period is often referred to as the ‘Miocene Climatic Optimum’ (Costeur et al. 2008). The Miocene Climatic Optimum is then followed by a sudden deterioration of the climate as a consequence of the reestablishment of the Antarctic ice cap (Domingo et al. 2009). The Late Miocene, is characterized by the aridification of the interiors or the continents (Bruch et al. 2007). During the Late Miocene a cooling phase starts between 13 and 14 Ma (Mosbrugger et al. 2007). This cooling phase is often attributed to a drop in CO2 levels, Mosbrugger however states that CO2 levels were not the driving force of the cooling phases. Between 15 and 13 Ma isotope curves show a gradual trend to heavier attributed to the cooling of deep ocean water, growing ice sheets on Antarctica and the beginning of Arctic glaciations (Mosbrugger et al. 2007). Figure 1.1 - Continental temperature curves (CMM) for Central Europe during the last 45 My in comparison with the global marine oxygen isotope record of (Zachos et al. ) adapted to the International Commission on Stratigraphy 2004 time scale (Mosbrugger et al. 2007) The European Miocene Environment A consequence of the aridification of the continents’ interior during the Late Miocene was the expansion of open landscapes. This spread of open landscapes also co-occurred with the spread of the C4-grasses, however no causal relationship has yet been proven (Molnar 2005). The expansion of the C4 plants was originally attributed to a drop in atmospheric CO 2 concentrations. However Pagani et al. (1999) argue that the CO2 concentrations showed an increase from a minimum value at 14 Ma (180 ppmv) up to values between 320 and 250 ppmv at the end of the Miocene (9 Ma). A drop in atmospheric CO2 levels as a cause for the C3-C4 shift has according to Strömberg (2011) fallen out of favor because during the Miocene CO2 levels remained stable and low. Both these researches are not in 8 correspondence with the popular belief that it was due to a drop in CO2 levels that the C4 plants expanded. Northern and central Europe stayed fairly forested throughout the Cenozoic. Grasses gained importance in Southern Europe and Asia Minor starting in the Miocene (Strömberg 2011). In Spain palynofloras point to open, arid steppe and woodland environment during the Early and Middle Miocene (Juménex-Moreno et al. 2007). For the Late Miocene Eronen et al. (2008) also maintain that there is a dry steppe like environment present especially in Spain. Van Dam et al. (2006) and Böhme et al. (2008) show in their researches that the Miocene climate had at least one or two extremely wet periods, the washhouse climates (Böhme et al. 2008). From 13 Ma both records that Böhme has reconstructed for Southwestern and Central Europe show a similar trend, evolving from a long dry period (13-11) Ma into a ‘washhouse climate’ (10.2-9.8 Ma). Strömberg (2007) describes habitats that seem to become more uniformly open towards the late Miocene (Strömberg 2007). According to Strömberg (2007) the Miocene environment can be described as a habitat with a mosaic of grass-dominated and more forest dominated habitats. Later Strömberg (2011) presents evidence, mainly based on pollen, that supports a dry environment for Europe and especially the eastern Mediterranean. This is in contradiction of the ‘washhouse climate’ for this period as suggested by Böhme et al. (2008). For the environmental patterns, as for the climatic patterns, it is noted by different authors that there were great regional differences (Domingo et al. 2009). During the Miocene punctual more regional patterns of faunal differentiations between eastern and western Spain are observed (Costeur 2008). This may explain the large differences between the many researches. Anchitherium taxonomy Within the order of Perissodactyla Owen, 1848 the Genus Anchitherium Meyer, 1844 belongs to the family of the Equidae Gray, 1821 and the subfamily Anchitheriine Leidy, 1869. The first Anchitherium fossil, found in 1825, was named ‘Paleotherium aurelianense’ by George Cuvier (Sanchez 1998). In this paper the following species of the Genus Anchitherium are defined; Anchitherium |-- A. clarencei Simpson, 1932 |--+-- A. aurelianense Cuvier, 1812 | |-- A. parequinum Sánchez et al., 1998 | `--+-- A. corcolense Inigo, 1996 | `-- A. castellanum Sánchez et al., 1998 `--+-- A. hippoides (author unknown) `--+-- A. matritense Sánchez et al., 1998 `--+-- A. alberdiae Sánchez et al., 1998 `--+-- A. cursor Sáncheze et al., 1998 `-- A. procerum Sánchez et al., 1998 And the Genus: - Sinohippus sampelayoi (Salesa et al., 2004) 9 Species included All species names printed in bold letters are species that are used in articles occurring in the literature part of this study. The most common species of Anchitherium appears to be A. aurelianense as described by Cuvier, 1812. Anchitherium gobiense Abusch, 1983 and Anchitherium nannoxerum Abusch, 1983 are included in the species Anchitherium aurelianense. The new species Anchitherium corcolense Inigo, 1996 also includes the species Anchitherium ezquerrae ezquerrae Abusch, 1983. The species A. ezquerrae ezquerrae is also identified in the material present at the Utrecht University archives. Salesa et al. (2004) proposed that Anchitherium sampeloy should in fact be grouped with the genus Sinohippus and thus be named Sinohippus sampeloy (Villalta and Crusafont, 1945). Due to discussion about whether Sinohippus is to be viewed as a separate species from Anchitherium (Salesa 2004 and Villalta et al. 1945) and possible occurrence in the region of interest the species Sinohippus sampeloy (Salesa, 2004) was also included. Also ezquerrae sampelayoi Abusch-Siewert, 1983 is included into Sinohippus sampeloy (Salesa et al., 2004). Hipparion taxonomy Within the Genus Hipparion (Hippotherium) much discussion has been going on about nomenclature and taxonomic inclusions and exclusions within the genus. Hipparion was named by Christol, 1832 and was assigned to Equidae by Kaup, 1833 and later to the tribus Hipparionini Quinn, 1955 by MacFadden (1998). Within this tribus also the genera Eurygnathohippus, Hippotherium, Nannihippus, Neohipparion and Pseudohipparion are currently included. Hippotherium was named by Kaup, 1832 and was later synonomized with Hipparion and Cormohipparion by MacFadden (1998) and Pesquero et al. (2006). The taxonomic discussion about the genus Hippotherium seems to be endless. Its diversification throughout Europe was very large. I will refer to the genus Hippotherium as a whole in this thesis and in the text will name the genus Hipparion. This is because the inclusion into Hippotherium is fairly recent and most authors still use the name Hipparion to indicate this genus. The species that are discussed in the papers used for this research (thus the species that occur in Europe mostly) are printed in bold in the following phylogenetic tree. In this paper the following species of the Genus Hipparion are defined: 10 Hipparion -genus-group | |?- Hipparion trampasense Edwards, 1982 |--o Merychippus sensu stricto | |-- M. insignis | |-- M. calamarius | `-- M. californicus |--o “Hipparion” group 3 [North American basal "Hipparion"] | |-- H. shirleyi | `--+-- H. tehonensis | `-- H. forcei `--o Cormohipparion Skinner & MacFadden, 1977 [Neohipparion, Notiocradohipparion] |-- Cormohipparion goorisi MacFadden & Skinner, 1981 [Neohipparion] |-- (Notiocradohipparion) Hubert, 1988 | |-- Cormohipparion (N.) plicatile (MacFadden, 1984) Hubert, 1988 | |-- Cormohipparion (N.) ingenuum (MacFadden, 1984) Hubert, 1988 | `-- Cormohipparion (N.) emsliei (Hubert, 1987a) Hubert, 1988 `--+?- Cormohipparion quinni Woodburne, 1994b [incl. C. sphenodus Woodburne et al., 1981] |-- Cormohipparion occidentale -species-group | `-- Cormohipparion occidentale (Leidy, 1856) [Neohipparion occidentale] Leidy, 1856, C. goorsi, C. quinni] `--+?- Cormohipparion theobaldi (Lydekker, 1877a) |?- Cormohipparion nagriensis (Hussain, 1971) `--o Hippotherium Kaup, 1833 [Old World “Hipparion”] |-- “Hippotherium” koenigswaldi Sondaar, 1961 |-- “Hippotherium” sebastopolianum |-- Hippotherium primigenium (von Meyer, 1829) |-- Hippotherium depereti Sondaar, 1974 |?- Hippotherium albertense (Hopwood, 1926) |?- Hippotherium baardi (Boné & Singer, 1965) |?- Hippotherium namaquense (Haughton, 1932) |?-+-- H. weihoense | `--+?- H. dermatorhinum | `--+-- H. coelophyes | `-- H. hippidiodus |--+--+-- “Hippotherium” catalaunicum (Pirlot, 1956) | `?- “Hippotherium” aff. “H.” catalaunicum `--+--+?- Hippotherium brachypus Hensel, 1862 | `-- Hippotherium giganteum Gromova, 1952 `--o Hipparion de Christol, 1832 |?- “Hipparion” melendezi Alberdi, 1974a |?- H. laromae Pesquero, Alberdi & Alcalá, 2006 |-- H. prostylum Gervais, 1849 `--+-- H. gettyi Bernor, 1985 `--+?- H. concudense Pirlot, 1956 |?- H. huangheense |-- H. campbelli Bernor, 1985 `-- H. dietrichi Wehrli, 1941 11 Anchitherium – Systematic Paleontology Order PERISSODACTYLA Owen, 1848 Family Equidae Gray, 1821 Genus Anchitherium Meyer, 1844 Anchitherium aurelianense, Cuvier 1812 Type locality; Montabuzard (France) Early Miocene (MN4) Locations; Dans les Sables de l’Orlénais; Chitenay; Neuville-aux-Bois and Chilleurs-aux-Bois (MN 3); Baigneaux, Chevilly et Maigrewille (MN 4b); Faluns de Pontelevoy et faluns d’Anjou (MN 5); La Romieu (MN 4b); Castelnau d’Arbieu (MN 5) in France; Buñol (MN 4) in Spain and Atzelsdorf (MN 9) in Austria. Diagnoses; (Abusch 1983) A. aurelianense is small and brachydont. The length of the upper cheek teeth P2-M3 varies between 105 and 119 mm. The length of the lower cheek teeth P2-M3 varies between 102 and 113 mm. In most of the M3 molars a crochet is present and is usually absent in the other upper cheek teeth (it is more abundant in Sansan). (extra) (Descprition of Daxner-Höck’s material from Atzelsdorf) The M3 is trapezoidal, the mesial width extends almost on third beyond the distal width. The lophodont paracone and metacone are integrated in the ectoloph. The bunodont lingual cones protocone and hypocone are continuous with the S-shaped protoloph and metaloph. Protoloph and metaloph contact the ectoloph. Protoconule and crochet are present. Parastyle and mesostyle are prominent. The tooth has a semi-continuous cingulum. The labial cingulum is weak, it extends from the parastyle towards the mesostyle. The pronounced distal and lingual cingula are continuous, the mesial cingulum extends in lingual direction towards the base of the protocone. Two pronounced conules are situated on the mesial and lingual cingulum. Anchitherium corcolense Inigo, 1996 Type locality; Córcoles (Guadalajara, Spain), Early Aragonian (MN4) Locations; Only found at the type locality Diagnoses; This species of Anchitherium has small postcranial bones and large teeth. The mean length of the upper cheek teeth P2-M3 is 125,89 mm and of the lower cheek teeth P2M3 129.3 mm. The maximal length of Mc3 varies between 163.2 and 180 mm and the maximal length of Mt3 between 160,3 mm and 181,5 mm. The upper jaw P1 tooth does not have a hypocone. The crochet is extremely infrequent on the upper cheek teeth (about 0,5 %) whose internal cingulum is very small and generally absent on molars. Proximal epiphyses of lateral metatarsals very broad related to their depth. The facets between internal metapodials and magnum or big cuneiform are small, but generally well defined. 12 Anchitherium castellanum Sánchez et al., 1998 Type locality; La Retama (Cuenca del Tajo, Spain) (MN 5) Locations; Only found at the type locality Diagnoses; The male canines are larger than the incisors. There is no crochet in the upper cheek teeth. There is a crochet present in 20% of all upper jaw M2 molars. In 20% of the upper jaw M3 molars there are styles and a valle central* present. There is no lingual cingulum present on the upper jaw M1 and M2 and a lingual cingulum is present on 30% of the upper jaw M3 molars. While in the premolars the lingual cingulum is almost always present, sometimes in 100% of all the samples studied. The lingual cingulum never appears on the lower jaw dentition. There is a high frequency of styles and the preflexid of the lower jaw dentition except for P2 where it is virtually absent. A style and a preflexid is present in all available lower jaw M3 samples. Differential Diagnoses; A. castellanum is different from A. corcolense by the greater length of the upper jaw M1 buccolingual; the absence of a lingual cingulum in the lower jaw dentition; the presence of a crochette and the absence of a short crochette*. A. castellanum differs from A. aurelianense in that their teeth are significantly larger. Towards the back the teeth become larger. And also they have a larger body size. *It is unclear how to translate ‘valle central’. ‘Valle distal’ means ‘Preflexid’ and ‘Valle Mesial’ means ‘Postflexid’ and these are names of lower jaw tooth forms. I have not found a translation for the apparent upper jaw tooth form ‘valle central’. Perhaps this is a new term coined by Sánchez et al. (1998). *’pliegue postfoseta corto’ translates as ‘short crochette’, this term probably is also coined by Sánchez et al. (1998). It is however unclear from the article what form he means by this ‘short crochette’. For A. matritense Sánchez also uses the term ‘long crochette’. Anchitherium matritense Sánchez et al., 1998 Type locality; Estacíon Imperial (Madrid, Spain) (MN 5) Locations; Puente de Valecas; Puente de Toledo; Estación Imperial; La Fuentecilla and La Cistérniga. Diagnoses; They have an overall large dentition. The male canines are about the same size as the incisors. A (short) crochette is present in all upper jaw teeth except the M2. The (long) crochette is only present in P2. A ‘normal’ crochette is present in the upper jaw P3, M1 and M3. The presence of a style and a preflexid in the lower jaw M3 is very low (8,33 %). Differential Diagnoses; The difference between A. matritense and A. corcolense and A. castellanum is the fact that the upper cheek teeth have a generally more square and more rectangular form. Also the lower relative size of the male canines is a difference. 13 Anchitherium cursor Sánchez et al., 1998 Type locality; Alhambra-Túneles (Madrid, Spain) (MN 6) Locations; Alhambra-Túneles and Arroyo del Olivar Diagnoses; This species has the largest dentition of all Anchitheriine species. The canines are similar in size to the incisors. The crochette is absent in the upper jaw dentition. The (short) crochette varies between 16-50% in the lower jaw dentition and the (long) crochette is present only in the M1. Anchitherium procerum Sánchez et al., 1998 Type locality; Paracuellos V (Madrid, Spain) (MN 6) Locations; Only present at the type locality Diagnoses; This species is big in body and dentition. The male canines are similar in size to the incisors. In the upper jaw P2 the style and central valley are absent. The buccal cingulum, the Metalophid and Hipolophid are absent in the lower jaw M3. There is no (long) crochette present in the upper jaw dentition, with the exception of P2 and M3. A (normal) crochette is present in the upper jaw M1. There is no style or preflexid in the lower jaw P4, M1 and M3. Differential diagnoses; A. procerum differs from A. cursor in its general size and the presence of a crochette. Anchitherium alberdiae Sánchez et al., 1998 Type locality; Paseo de las Acacias (Madrid, Spain) (MN 5) Locations; Paseo de las Acacias, Peñuelas and la Hidroeléctrica (Madrid) Diagnoses; This species is medium sized but has a large dentition. Its male canines are similar in size to its incisors. The size of the canines would be comparable to those of a dog. The authors note that none of the complete jaws contains a P1, they conclude that there may have been an advanced reduction of the P1 in this species. Styles appear in the preflexid of the M3 with high frequency (71,42 %). Differential diagnoses; The P1 in this species is significantly smaller than the contemporary species like A. matritense. 14 Anchitherium parequinum Sánchez et al., 1998 Type locality; El Terrero (Villafeliche, Spain) Locations; El Terrero, Valdemoros and Ambos en Villafeliche Diagnoses; This is a very small and slender species (no comments on the dentition) Differential Diagnoses; this is smallest of all species by comparison. Sinohippus sampelayoi (Salesa et al., 2004) Type locality; Nombrevilla-1 (Calatayud-Daroca Basin, Zaragoza, Spain) Locations; Only in type locality Diagnoses; (Villalta and Crusafont 1945: 78, Diagnoses of Anchitherium sampelayoi) “terminal derived Anchitherium of large size, with marked tendency to hypsodonty, basal cingulum almost obsolete, mainly in the juvenile teeth; more evident homeodonty than in the primitive forms, milk teeth highly reduced, special shape of d2”; additionally: upper premolars with marked buccal cingulum and absence of lingual cingulum, hypostyle without connection with the metalophe, and absence of crochet. Remark; The lower premolars of the Nombrevilla-1 species are very similar to the proportions of the remains from Sinohippus from another location. This relative reduction if not present in Hypohippus and Megahippus. This reduction of the premolars is sufficient to distinguish between the North American and the Eurasian giant Anchitheriinae (Salesa et al. 2004). 15 Distribution Anchitherium first enters Europe during the Late Early Miocene. The first Anchitherium fossils have been found in Chitenay; Neuville and Chitelleurs (France) (Abusch and Siewert 1983) and in Merkur (Czech Republic) and Wintershof-West (Germany) (Bruijn et al. 1992). The first occurrence of Anchitherium in Spain is at the geological site of Agreda (Soria, Spain MN 3) (Sanchéz 1998). Anchitherium is part of the second of four (equid) migration waves from North America via Asia that took place during the Early Miocene (Salesa et al. 2004). The third migration wave that took place during the Early Middle Vallesian includes the North American tridactyl equid Hipparion (Salesa et al. 2004). This second migration into Europe is marked as the ‘Hipparion date’ and coincided roughly with the decline and extinction of the genus Anchitherium. Both genera show co-occurences at several different sites in Europe so we know that both genera co-excisted in Europe for at least a while. Cooccurrence of Anchitherium and Hipparion is observed at a few localities of basal Vallesian (MN 9) age from Atzeldorf; Mogersdorf; Mariathal and Radlebrunn (Austria) Geisenberg (Germany) (Höck 2009) also in Nombrevilla-1 (Spain) (Villalta and Crusafont 1945); Doué-laFontaine and Soblay (France); Holzmannsdorfberg (Austria), Ucak (Turkey). Between its initial arrival in Europe and its extinction roughly 10 million years later the Genus Anchitherium was widespread throughout Europe and Asia. From the literature research it can tentatively be concluded that one of the most common species in southern Europe, France and the area of interest Spain appears to be Anchitherium aurelianense. A. aurelianense has been described from more than 50 sites in Spain alone (Inigo 1997). By authors Abusch, (1983) and Inigo (1993) this species is only recognized at the site of Buñol (Valencia, Spain). These two authors classified the anchitheriines from many other sites of the internal basins of the Iberian Peninsula as Anchitherium ezqeurrae (Meyer 1844), and not as A. aurelianense. A. ezquerrae ezquerrae has by Inigo (1996) been reassigned to A. corcolense. Geological setting Anchitherium Anchitherium first appeared in North America in the Early Miocene and entered Europe via Asia. This migration route also becomes apparent in the distribution of geological sites with Anchitherium finds worldwide (figure 2.1). Anchitherium entered Europe during the middle Orleanian, MN 3 and becomes extinct during the Vallesian, MN 9 (Kaiser 2009, Forsten 1991). Zone MN 3 is characterized by the establishment of land connections in south-eastern Europe. The co-called ‘Gomphotherium landbridge’ (Rögl 1999) links the Eurasian and African continents (Costeur and Legendre 2008). And several taxa immigrated into Europe. Anchitherium however, unlike Hipparion much later, did not use these landbridges to migrate into Africa. The oldest geological finds are located in North America but will not be discussed here since the focus lies on European and especially Spanish localities. 16 Figure 2.1 – Worldwide distribution of the genus Anchitherium (Fortelius et al. 2009) The first occurrence of Anchitherium in Europe is during the late Ramblian (Early Miocene). The first Anchitherium fossils have been found in Chitenay; Neuville and Chitelleurs (France) (Abusch and Siewert 1983) and in Merkur (Czech Republic) and Wintershof-West (Germany) (Bruijn et al. 1992). The first occurrence of Anchitherium in Spain is at the geological site of Agreda (Soria, Spain) (Sanchéz 1998). Following are the different localities with Anchitherium finds that provided the data for the articles that were used for the literature research. The localities used in the practical part of this thesis will be discussed separately in another chapter (Practical work). Per locality it will be discussed in what articles the locality is named, if possible how old it is and which species of Anchitherium was found there. Córcoles- (Guadalajara, Spain) According to Inigo (1997) a new Anchitherium species was present at the Spanish site of Córcoles, Anchitherium corcolense. This new species shows a primitive anatomy with respect to A. aurelianense and A. ezquerrae. The species is related to a dry forest environment. The site dates from the early Aragonian (the Spanish name for the Orleanian period, figure 2.3). The local periods have not all been dated to the same time period. Atzelsdorf- (Paleo-Danube delta in Austria) Höck (2009) describes the Anchitheriine fossils found at Atzelsdorf as A. aurelianense. This ancient delta is of Vallesian age and is therefore also one of the localities in which a cooccurrence with Hipparion can be observed (in zone C of the early Pannonian). The Pannonian is a local name for the Vallesian period. 17 Nombrevilla 1- (Spain) (Salesa 2004) propose that the species Anchitherium sampelayoi, found at the site Nombrevilla 1, identified by (Sanchéz et al. 1998) should be included within the genus Sinohippus and therefore be named Sinohippus sampelayoi. Samosaguas- (Madrid basin, Spain) The geological site for Anchitherium cursus is the site of Samosaguas in the Madrid basin (Spain) (Domingo et al. 2009). Sandelzhausen- (Germany) At the geological site of Sandelzhausen in Germany the Anchitherium species found there has been identified by many authors as A. aurelianense (Eronen et al. 2009) (Kaiser 2009) Other localities of samples used by Eronen et al. (2009) in their research are Puente de Vallacas (Spain) with Anchitherium aurelianense and Anchitherium sp. Alhambra (Spain) with Anchitherium sp. and La Retama (Spain) with Anchitherium castellanum. It is interesting to note that A. aurelianense as well as an unidentified species of the genus Anchitherium have been noted to be present at the site at Puente de Vallacas in Spain. Janis (2008) states that like in the present day it is also unlikely for fossil assemblages to contain more than one species of horse. This might indicate that the unidentified species may also be A. aurelianense. Or it may be an indication that a cooccurence of different species of horse in a fossil assemblage isn’t so rare after all. Hipparion The mammalian ‘Hipparion Fauna’ was established in the early Vallesian and existed for more than 7 million years up to the middle of the Pliocene. The dominant species of this fauna was Hipparion that migrated from North America (Vislobokova 2006). The Hipparion fauna populated the East Alpine Region alongside Middle Miocene forms that survived well in wet situations (Vislobokova 2006). The earliest Hipparion horse fossils, Hippotherium primigenium (previously Hipparion primigenium) were found in the Vienna Basin at the sites Hovorany and Gaiselberg (Bernor et al. 1988, Rögl and Daxner-Höck, 1996). There is a general consensus that this species appeared in this territory not earlier than 11.5 Ma (Bernor et al. 1988, Vislobokova, 2006). A rather rapid dispersal of Hipparion and the formation of the Hipparion fauna were promoted by geographical conditions that existed at the boundary of the Middle and Late Miocene and during the late Miocene (Vislobokova 2006). As becomes apparent from the world map (figure 2.2) showing geological finds generated by the Palaeobiological database the Hipparion horses were much more wide spread than the Anchitheriinae. From this map it becomes clear that the Hipparion migrated along the same route as Anchitherium via Asia into Europe. Hipparion also made it into the Southern part of Africa and South East-Asia which Anchitherium never did. 18 Figure 2.2 – Worldwide distribution of the genus Hipparion (Fortelius et al. 2009) Biochronology For continental fossil finds during the Miocene and Pliocene of Europe the MN-zonation (Mammalian Neogene- zonation) is often applied. Because continental fossils are difficult to date and also hard to relate to marine records, MN-zonations can be used to give an indication of the time a certain species lived in an area. These zonations are defined through reference faunas and are often bordered by migrations into or out of a certain area or extinctions of certain species. There are sixteen consecutive MN zones spanning the Miocene and Pliocene (MN 1 through MN 17; MN 7 and MN 8 have been joined into MN 7/8) MN 1 being the oldest and MN 17 the youngest fauna zonation (Bruijn de et al. 1992). Due to redefinition of the boundary between the Neogene and Quarternary periods, MN 17 is now in fact considered a Quarternary biozone. The MN zonations of interest to this research are the zonations MN 3 to MN 9 which roughly correspond to 20-10 Ma ago (figure 2.3) MN 3 is (partly) defined by the introduction of Anchitherium and MN 9 is (partly) defined by the introduction of Hipparion. 19 Iberian Biozonation The biozonations vary per different country. Apart from the MN Zonation the Iberian biozonation is also very relevant for this study. I have added a figure (figure 2.3) by (Hordijk, 2010) showing the MN zonation relative to the Spanish version, the Iberian biozonation. Added are two different age calibrations by (Daams et al. 1999 and van Dam et al. 2006). Figure 2.3 The Iberian biozonation (Hordijk 2010) 20 Central Paratethys biozonation Figure 2.4 shows the Central Paratethys and the Eastern Paratethys biozonation. Of interest to this research is mainly the Central Paratethys biozonation because it is the local zonation for the Austrian faunas including Hipparion and Anchitherium. Most important in the Central Paratethys biozonation is that the continental period ‘Vallesian’ is called the ‘Pannonian’. It is named after the Austrian Pannonian basin where it is defined. The articles about the German localities use the MN biozonation. Figure 2.4 – The Central Paratethys biozonation (Vislobokova 2006) 21 Chapter 2 – Literature Research and Methods Evolution and adaption of Anchitherium If Anchitherium changed its diet morphological adaptations were needed. These adaptations would probably have consisted of specialization of the molars and maybe of the digestive system. This latter part of the animal does not fossilize very well, the teeth however are often the body parts of large mammals that have the highest fossilization potential (Forsten 1991). There are many different methods to deduce a fossil species living environment and diet. Micro and mesowear patterns on fossil teeth are one possible approach (Kaiser 2009, Fortelius and Solounais 2000). Another method is to extract carbon isotope data from tooth enamel (MacFadden 1994) or bones. Forsten (1991) bases her conclusions about Anchitherium’s diet on its general body size compared to the size of its teeth. In this chapter the methods used to deduce what the diet of Anchitherium was will be summarized. And also the research dealing with Anchitherium’s possible evolutionary adaptations to a changing world will be discussed. Changes in body and tooth size Forsten (1991) states that Anchitheriums never became hypsodont, or showed signs of ‘incipient hypsodonty’ in contrast to the later research of Eronen et al. (2009). Anchitherium remained morphologically conservative and according to Forsten (1991) this may have been the reason for its demise. The only morphological response that the Miocene Anchitherium showed was a slight increase in occlusal surface, caused by an increase in tooth size. Inigo (1996) describes Anchitherium corcolense found at Corcoles in layers from the lower Aragonian (Orleanian) (MN3 – MN4). He describes this species as macrodont and defends that this species was already separated from the most primitive microdont Anchitherium. Forsten (1991) states that large teeth are derived in Anchitherium. She considers the species A. aurelianense from Buñol as primeval and states that dental size increases abruptly in the Spanish Anchitheres from the middle Orleanian (in Iberian biozonation: Aragonian) with A. ezquerrae. Inigo later argues that the species A. corcolense from the lower Orleanian is older than the specimens from Buñol and is macrodont. Thus according to Inigo (2001) these Anchitheres were already separated from the most primitive forms earlier than Forsten suggests. And macrodont Anchitherium species may have already been present on the Iberian Peninsula from the early Orleanian, early Miocene (MN3). Forsten (1991) concludes that the dental morphology and body size of the Middle and Late Miocene Anchitherium suggest that it was a browser and perhaps a mixed feeder. If this is the dental morphology that Inigo (2001) also refers to as macrodont teeth this may be an indication that there are already signs of Anchitherium’s mixed feeding in the early Miocene (MN3) when it first set foot on the European continent. 22 Micro- and Mesowear pattern analysis Kaiser (2009) bases his conclusion that Anchitherium was a ‘dirty browser’, on the mesowear patterns on fossil teeth. This method was initially developed by Fortelius and Solounias in 2000 and has proven a powerful tool for reconstruction palaeodietary adaptations. Unlike the microwear method that may suffer from the ‘last supper syndrome’ (Solounias 1994) the mesowear patterns reflect long term diet and is therefore much more reliable. Kaiser states that the mesowear pattern found on Miocene Anchitherium teeth from in Sandelzhausen (Germany) indicates a diet of mixed feeding. However, he acknowledges that grit incorporated into a browsers food may have the same mesowear signature as a mixed feeder (Williams and Kay 2001). For this reason Kaiser has aptly named his article ‘Anchitherium, a dirty browser’, hinting towards the possibility that Anchitherium browsed for leaves in places with a high dirt content, such as near a river. Also Kaiser concludes that Anchitherium was in fact probably an opportunistic mixed feeder instead of a pure browser. Tooth enamel and bone Isotope analysis Meso- and microwear analysis on the teeth of fossil species has provided clues about the Anchitheriine diet (Eronen et al. 2009, Kaiser 2009. DeMiguel et al. 2011). Apart from mesoand microwear analysis and general crown height, isotope analyses of bones and tooth enamel can also provide valuable clues about fossil species and their diet (Domingo et al. 2009, MacFadden et al. 1994). Carbon-isotopes The carbon isotopic composition of bones and tooth enamel of herbivore mammals records the isotopic composition of the plants they ate and can therefore be used to distinguish between the different types of palaeodiets. There are 3 different photosynthetic pathways, C3, C4 and CAM (Crassulacean Acid Metabolism). The CAM Pathway is mainly used in succulents and therefore not relevant for looking at the Anchitheriine palaeodiet. The C3 photosynthetic pathway is much older than the C4 pathway and includes about 85% of all terrestrial plants including trees, most shrubs and high latitude or high elevation grasses. About 10% of the terrestrial plant biomass today use the C4 pathway, these include mainly tropical and temperate grasses that are adapted to climates with higher seasonality and with relative greater aridity than C3 plants (Domingo et al. 2009, MacFadden et al. 1994). When trying to determine whether the Anchitheriine diet contained grasses it is interesting to look at the isotopic composition of its teeth and bones. C3 plants discriminate more against the heavy isotope 13C during photosynthetic fixation of CO2 compared to C4 plants. This results in different carbon isotopic compositions of the different plants. A relation has been proposed between the enrichment in 13C of tooth enamel and diet by Domingo et al. (2009). Lower values would be more indicative of more browsing behavior whereas higher values indicate a diet of drought resistant plants of grasses. If the isotopic signal indicates that the animal consumed C4 plants it is possible that it ate grass, because the C4 pathway is only present in grasses. It may also mean that its habitat was relatively dry. However if there is no isotopic signal indicating the consumption of C4 grasses this does not mean that Anchitherium did not eat grass because C3 grasses were very abundant. 23 Domingo et al. (2009 B) find higher d13 C for the Anchitherium species A. cursor and also in the extant ruminant species used for their research. They tentatively conclude that these high values may be an indication that these species had a diet selection towards plant species with a higher evaporation rate. They do not say that this means that they ate C4 grass, but they plot further away from the pure plant eating, thus browsing species. For the ruminants the higher isotopic value may also be a result of their digestive tract, ruminants produce high quantities of methane during their digestion as a result of foregut fermentation (Domingo et al. 2009 B). For Anchitherium however this higher value may yet be indicative of a diet of plants with a higher evaporation rate, and not a diet of juicy leaves of a pure browsing animal. Domingo et al. (2009 B) do not directly conclude that Anchitherium’s diet contained C4 grasses. In another article based on isotopic research on Hipparion dental enamel from the Terual-Alfambra region Domingo et al. (2009. A) do speculate about Hipparion’s diet. The isotopic research on Hipparion dental enamel has yielded d13C values typical of a diet based on C3 plants (Domingo et al. 2009 A). It is unfortunate that the Teruel-Alfambra paleontological sites span an age range between approximately 10.9 and 2.7 Ma because the low values are found in dental enamel of Hipparion horses older than MN14. It is during MN14 that Domingo et al. (2009 A) note a significant increase in the isotopic d13C value that may be indicative of a slight increase of the consumption of C4 plants. So it would appear as if Kaiser (2009) may be correct in stating that Hipparion changed its feeding habits upon entering Europe from grazing to mixed feeding or even browsing . Because from this isotopic data it appears as if Hipparion turned (back?) to the grazing C4 niche much later. Oxygen isotopes By analyzing d18O values from herbivore tooth enamel it is possible to differentiate between obligate drinkers (often grazers) and non obligate drinkers (browsers). Because grazers get most of their water from drinking whereas browsers obtain their water via the diet of plants that they eat. Domingo et al. (2009) do not interpret their oxygen isotope data in this way. The values to plot their measurements against are absent so the Anchitherium tooth oxygen isotopic data in the form of a water intake proxy cannot be interpreted as such. Ba/Ca ratio According to Domingo et al. (2009 B) the, enriched Ba/Ca ratio shows that Anchitherium cf. cursor had a more mixed feeding strategy. 24 Hypsodonty Apart from the question whether or not Anchitherium has adopted evolutionary changes for eating grass by becoming more hypsodont (Eronen et al. 2009) and why, another important question is; ‘Is hypsodonty really an adaptation for eating grass or more abrasive foods?’ Hypsodonty has evolved many times during the history of vertebrates and in many different lineages. The evolutionary advantage of hypsodonty is obvious, since tooth wear is a proximate cause for senescense (Eronen et al. 2009). Hypsodonty has also evolved along many rodent lineages in order to cope with the hard nuts and seeds these animals eat and do cut through tough roots while digging holes. Hypsodonty has evolved numerous times within mammals in general (Janis 1994). Hypsodonty is not the only adaptation to eating abrasive foods. Along with hypsodont teeth other changes in tooth morphology that increased chewing effectiveness also occurred. Examples of this are increased tooth surface (Forsten 1991) and increased complexity of enamel folding and development of cementum (Evander 2004, Strömberg 2006). However according to Strömberg (2006) these dental properties often seem decoupled in horses. The general accepted theory for grazing animals is that they have developed hypsodont teeth in order to cope with the abrasive phytoliths (silica particles that make grass an extremely abrasive food to eat) that are incorporated into the structure of grass (Strömberg 2005). The general idea in horses and ruminants is that hypsodonty indicates a diet based mainly on grass and therefore that the spread of hypsodont ungulates must have coincided with the spread of the grasslands (Retallack 1983). However this idea is topic of debate. According to Janis (1994) hypsodonty is unlikely to be a specialization for grazing. She notes this in the ungulate family. ‘Grazing is a fairly recent specialization among ungulates although extensive grasslands appeared around 25 Ma - and along with them hypsodont ungulates – real specialized grazers did not appear until around 10 Ma and most of these grazers are of Plio-Pleistocene age’ (Janis 1994).Fortelius et al. (2002) base their climatic reconstruction of Eurasia on the level of hypsodonty. They state that in general a higher hypsodonty rate corresponds to an environmental parameter that they call ‘generalized water stress’. They link hypsodonty more to a dryer environment than an increase in grassland and open vegetation. Also according to Strömberg (2011) the evolution of herbivores adapted to grasslands did not necessarily coincide with the spread of open-habitat grasses. Modern faunal studies indicate that hypsodonty correlates more closely with habitat openness, feeding height, and levels of aridity than with proportion of grass consumed (Eronen et al. 2010). Abrasiveness of the food of grazing animals is also not necessarily caused by the phytolith particles in the grass. It may be due to: - An increase in grid uptake, caused by grazing near river beds. Extrageneous dust covering the grass, often desert sand. An overall increase in food uptake by the animal because it became bigger or the food source became less nutritious (Strömberg 2005, Eronen et al. 2009). 25 Another reason why the evolutionary adaptation of hypsodont teeth is not necessarily a consequence of the spread of grassland is the adaptive lag that is observed by (Strömberg 2001). The North American grasslands already developed during the Oligocene (Retallack 2001) and this was 5 million years before the development of hypsodonty in ungulates on the North American continent (Strömberg 2001). According to Janis (1994) it is highly unlikely that an adaptive trait develops after the need, because in this case hypsodonty is essential to the grazing animal. The strange situation that now appears can be solved by a principle that was coined by Gould and Vrba (1982) as ‘exaptation’. A trait may develop shaped by selection to serve a different function than they are presently serving. Thus hypsodonty may have developed earlier, or as in this case later serving a different purpose but also seemed to increase fitness for grass eating animals. That is the reason why we connect hypsodonty to eating grass, this may however not be its evolutionary origin at all. The hypsodonty discussion learns us that we should not base our conclusions about the diet of, for example, fossil horses on the level of hypsodonty of their teeth, and that the incipient hypsodonty of Anchitherium may not have been a specific adaptation for eating grass. A brachydont animal is extremely unlikely to be anything but a browser, but a hypsodont species may have a variety of diets (Janis 1994). Also the paleoenvironmental reconstruction of the spread of the grasslands can not necessarily be deduced from the spread of more hypsodont species for an increase in grass consumption is probably not the, or not the only cause for the evolutionary adaptation of hypsodonty. The most viable hypothesis according to Strömberg (2001) is that hypsodonty is to be connected to feeding in an open habitat on grass and or dust and grid contaminated food. Climate and environment In chapter 1 an overall climatic and environmental sketch was drawn of the Early to EarlyLate Miocene. Researchers agree that there are large local differences in environmental conditions as well as climatic effects such as rainfall. This is called ‘local endemism’ and is especially strong in south eastern Europe (Mosbrugger et al. 2005, Costeur and Legendre 2008). Also conditions have differed greatly throughout course of the Miocene. In chapter 2 climatic and environmental reconstructions and the proxies used will be discussed in order to bring some detail to the overall sketch of the supposed dry ending of the Miocene which brought about the expansion of open landscapes and disputably the spread of the C4 grasses (Molnar 2005). When looking at the Miocene in greater detail it becomes apparent that the dry periods were most likely set apart by wet periods and even a period at the end of the Miocene that Böhme et al. (2008) refers to as the Late Miocene ‘washhouse climate’ (see page 9). There are many different ways to reconstruct palaeoclimate and environment: micro- and macrofossil fauna, pollen and fossil flora, dental enamel isotopes, dental wear and level of hypsodonty (Fortelius et al. 2002). All of these different methods lead to, sometimes very different, results and conclusions about past climate and environment. 26 Climate reconstructions and proxies An important point on which Eronen et al. (2009) base their assumptions of the adaptation of Spanish Anchitherium is the fact that Spain was the first region to experience aridity during the Early Miocene of Europe, and that the region where the ‘contemporary’ German specimens are found remained humid during that time. In order to prove or disprove this part of Eronen’s hypothesis it is important to study the climatic conditions in Europe, and especially Spain during the Early Late Miocene. Overall the Late Miocene is seen as a period that is characterized by the aridification of the interiors of the continents (Bruch et al. 2007). Many researchers agree however that there may have been large regional differences (Mosbrugger et al. 2005, Costeur and Legendre 2008). Fossil fauna Eronen and Bruch support an overall aridification of the continents however van Dam et al. (2006) and Böhme et al. (2008) show in their researches that the Miocene climate had at least one or two extremely wet periods, the washhouse climates (Böhme et al. 2008). Böhme bases her estimates of precipitation on the ‘ecophysiological structure of herpetological assemblages’ (amphibians and reptiles). Both records reconstructed for Southwestern and Central Europe show a similar trend, evolving from a long dry period (1311) Ma (MN 7-8) into a ‘washhouse climate’ (10.2-9.8 Ma) (MN 9). They base their conclusions on two records taken from two locations spatially far apart. One from the Calatayud-Daroca basin in Spain (from two different continental sequences) and one from several locations in the Paratethys region. Their proxies show that precipitation was several times higher than present. From 9.7 Ma onward their records show that the climate became dryer again and record a second washhouse climate between 9.0-8.5 Ma. The first supposed wet climate in South Europe is of special interest for this research because it roughly coincides (10.2 -9.8 Ma) with the extinction of Anchitherium and for which at least one of this thesis’ hypotheses states that this may have been caused by climatic change. It contradicts Eronen’s claim that Spain was dry during this period of the Miocene. Böhme is not the first researcher arguing an increase in precipitation rates during the Early Late and Late Miocene. Earlier van Dam (2006), who bases his research mainly on rodent faunas from Spain, argues that after the relatively dry conditions of the Middle Miocene (16-13 Ma), an increase in the precipitation rate took place between 13 and 11 Ma (MN 7-8), reaching a maximum at about 11-10 Ma (MN 9). Dental isotope composition The isotopic composition of the dental enamel of fossil species can be a proxy for many different things. Earlier in this chapter the use of Carbon isotopes to reconstruct diet has been discussed. The water that the animals drink is often meteoric water from rivers and lakes. Thus the isotopic composition of the water can be used (when analyzing only one genus) as a proxy for palaeotemperatures and the evolution of past climates. The d18O values measured in the dental enamel of Hipparion horses from Spain by (Domingo et al. 2009) show an increase in d13C values during the period after the Miocene Climatic Optimum 27 indicating an increase in aridity (MN 7). Their data also shows a gradual increase in d13C CO2 values starting MN9 to MN16. This may be related to an increase in the mean annual temperature of approximately 2,1 degrees Celsius. Dental wear Domingo et al. (2009)’s findings are contradicted by the data from DeMiguell et al. (2011). They base their data on the dental wear of Spanish ruminants. He uses a combined approach of dental microwear and mesowear, and uses the reconstructed diets as environmental and climatic proxies. Their data show a transition from dry conditions in the Late Early (MN 4) and Middle Aragonian (MN 5) that persisted into the Late Aragonian (MN 6, MN 7/8) to more humid conditions during MN 7/8. However there are some problems however with the microwear method, this method suffers from the so called ‘last supper syndrome’ (Solounias 1994). Level of hypsodonty Fortelius et al. (2002) in their climate research formulate a new quasi-quantitative proxy. They link the level of hypsodonty to a environmental condition that they term ‘generalized water stress’. According to Fortelius (2002) the Early Miocene shows a pattern of faunas dominated by brachydont species except for two areas of early increase in crown height; central Asia and the Iberian Peninsula. They conclude that the Early Miocene remained green with only the slightest evidence of incipient aridity. They also note that the intervals that show the incipient hypsodonty are all from the later part of the Early Miocene. The Middle Miocene shows slightly more evidence of increasing aridity. The Earlier Late Miocene however shows a major strengthening of the hypsodonty in the present day Mediterranean region and in east-central Asia. If Fortelius’ climatic reconstructions are correct this would imply that dry conditions in Spain occurred later than the Early Late Miocene. This partly undermines Eronen’s (2009) claim that the Spanish Anchitherium responded to the conditions on the Iberian Peninsula as the climate changed during the Early Miocene. This new proxy is controversial because it has two major problems. First of all the link between hypsodonty and climatic change is not as well established as Fortelius (2002) presents it. There is still much discussion about hypsodonty and diet (Strömberg 2009). Linking diet to environment and even climate is a dangerous step because other species that have already evolved a high level of hypsodonty may move into an area making it appear drier. Fortelius acknowledges this problem. Another important remark on Fortelius’ research is the fact that the results, when including horses in the hypsodonty index faunas, change enormously. He notes this in his paper and states that he does not have an explanation for the large deviations in data when horse hypsodonty levels are included. Pollen and flora The marine realm is generally understood quite well, but the continental climate patterns are not (Bruch et al. 2007). Earlier Mosbrugger et al. (2005) conclude that the European continental climate correlates well with the oxygen isotope record from the marine environment. Mosbrugger et al. (2005), who base their research on extensive floral data, 28 conclude, apart from some minor variations, that the Mean Annual Precipitation (MAP) levels were commonly on a high level (of more than 1000 mm/y) throughout the Central European Cenozoic. During the Late Miocene and earliest Pliocene he finds that MAP remained constant at a level of ca. 1250 mm/y to then significantly drop only during the Zanclean (MN 13, MN 14). However for Europe and the eastern Mediterranean region Strömberg (2011) presents evidence, based on pollen data, that supports a dry environment. Environmental reconstructions and proxies As a consequence of the aridification of the interiors of the continents there was a supposed expansion of open landscapes (Bruch et al. 2007). There is much discussion as to if this ‘overall continental aridification’ can be applied to all areas. Many researchers agree that large regional differences should be taken into account, especially when looking at the evolution and adaptation of large vertebrate species (Salesa et al. 2010). The aridification of Europe during the Late Miocene supposedly started on the Iberian Peninsula (Eronen 2009) and it brought about the spread of open landscapes which came with the invasion of the C4 type grasses. The adaptation that Anchitherium supposedly underwent was one for eating more abrasive foods, and C4 grasses would qualify as more abrasive foods because of the phytolith (silica) content. The timing of the spread of C4 grasses is therefore very important for this thesis. There is much discussion about the exact timing of the spread of C4 grasses. The Late Miocene is often seen as the period that witnessed the extreme radiation of this grass type. For Anchitherium to so radically change its feeding habits that it required evolutionary adaptation it is important to research the timing of the C4 type grasses becoming the dominant vegetation in open landscapes. Pollen, Flora Many researchers argue that C4 grasses became the dominant open landscape vegetation at the end of the Miocene (Janis 1994 and Domingo et al. 2008). Janis writes in her textbook that around 8 Ma there was a shift in grass type from C3 to C4 type grasses. Grasses gained importance in Southern Europe and Asia Minor starting in the Miocene. In Spain palynofloras point to open, arid steppe and woodland environment during the Early and Middle Miocene (Juménes-Moreno et al. 2007). This would support Eronen (2009) in his claim that in Spain the arid steppe and woodland environment evolved earlier than in the rest of Europe. However, Eronen times this event of aridification much later than Juménes-Moreno et al. (2007). Strömberg (2011) states that Northern and central Europe stayed fairly forested throughout the Cenozoic. Isotopic composition Several studies in paleosoil carbonates (Quade et al. 1992 and Morgan et al. 1994) place the dominance of the C4 grasses during the Late Miocene and Early Pliocene (between 7- 5 Ma). Even later than the Late Miocene in the studies of Janis (1994) and Domingo et al. (2009 B). The isotope data extracted from paleosoils show a sudden change in d13C between 7 and 5 Ma. It needs to be noted that the two investigations that she uses to support this claim are from researches done in Pakistan and Kenya. Macfadden (1994) finds that the isotopic value 29 of the grazing species that lived during the Late Miocene he researched indicates a C3 dominated diet. The fact that these animals are grazing species indicates, according to Macfadden, that the dominating grass vegetation was still C3 and not C4 grass. This conclusion is consistent with the isotopic shift, supposedly indicative of the shift from C3 to C4 grass domination, that Quade et al. (1992) and Morgan et al. (1994) find between 7- 5 Ma (Miocene Pliocene boundary). Domingo et al. (2009 A) note in their research on Hipparion dental enamel that there is a significant increase in the isotopic d13C value around MN14MN15 (5-4 Ma) thus in the Pliocene. This increase may signify an increase in the consumption of C4 plants. And may be indicative of an even later spread of the C4 grasses in Europe than was previously thought (data from the Teruel-Alfambra region). CO2 The overall consensus is that the predominance of C3 versus C4 plants depends on temperature and CO2 partial pressure (Ehleringer and Pearcy 1983, Ehleringer and Monson 1993). Ecosystems dominated by C4 plants are those with high temperatures and aridity. These are also the ecosystems that show low CO2 partial pressure values because C4 plants can concentrate the CO2 in their leaf cells before carrying out photosynthesis (Domingo et al. 2009). Thus initially the spread of the C4 plants at the end of the Miocene was attributed to a drop in CO2 concentrations during the Late Miocene. However Pagani et al. (1999) argued that the CO2 concentrations showed an increase from a minimum value at 14 Ma (180 ppmv) up to values between 320 and 250 ppmv in the later Miocene (9 Ma). A possible conclusion from this can be that if the expansion of the C4 grasses was triggered by the lower CO2 values it should have occurred at the beginning of the Miocene. The presumed drop in CO2 levels at the end of the Miocene has often been seen as a cause for the spread of the C4 grasses, but if this drop never occurred this handheld is also lost. Fauna The macrodont species Anchitherium corcolense is, according to Inigo (1996), better adapted to a drier environment. This species lived during the Aragonian (MN3 and MN4) in Spain. Inigo associates this species with the environment ‘open woodland’ or ‘woodland savannah’. Also found at Corcoles, is the rhinoceros Hispanotherium matritense, a grazer. However Inigo does discuss that Anchitherium is more abundant in the layer that is associated with less dry environmental conditions. Höck (2009) recognizes the fact that Anchitherium aurelianense found in MN 3 layers from Austria is a typical brachydont species, which may be indicative of a woodland environment. The faunal evidence by Inigo and Höck could point out that Spain was already a drier area of Europe when Anchitherium first arrived during MN 3. 30 Chapter 3 - Practical research Introduction The practical part of this thesis will mainly consist of morphological research on Anchitherium fossils from localities in Spain (figure 3.1) Like most fossil horse material from the New as well as from the Old World (Forsten 1991) the material consists mainly of isolated teeth. Measurements on different species as done by: Inigo (1997), Sanchez (1999), Hernández Fernández et al. (2003), Alberdi et al. (2004), Salesa et al. (2004), Daxner-Höck and Bernor (2009) will be repeated and compared to the data from the literature. The specimens used in the research are from the Utrecht University paleontological collection. Some specimen have been determined on species level and all specimens, bone and teeth, belong to the genus Anchitherium Meyer, 1844. I will discuss the specimens per locality. The morphology will be examined and the size of the teeth will be measured in order to determine (wherever possible) the specimen on species level. The original idea of this practical research was that micro- and mesowear analysis would be attempted. It turned out however that Utrecht University did not posses research facility needed for this kind of research and also that the sample sizes were too small for decent statistical analysis. Isotopic research was not performed because of the small amount of material and the fact that the fossils needed to be destroyed partly or entirely for this type of analysis. Figure 3.1 Localities 31 Methods The measurements on the molars have been performed using a precise measuring device. Measurements have been done multiple times in order to minimize room for error and have been noted in mm (and 0,1 mm). Of the molars the anterior width, posterior width and length have been measured on the widest part of the tooth. Length has also been measured on the longest part. (figure 3.2) Technical practical problems Measuring problems None of the authors included the measuring method of the molars in their article. Therefore a problem that was faced was the fact that for species determination all authors used one single value for width as well as for length of the teeth. During the first attempt, the mean value of the posterior and anterior width of the samples was calculated and plotted in the different graphs. Later it turned out that it was standard practice, unless indicated otherwise, to always measure the widest part of the tooth. All graphs have been corrected for this different approach. Nomenclature Another problem that was encountered was that of the nomenclature. The upper cheek teeth of horses are among the largest and most complex of mammalian teeth. To make matters worse, terms introduced by one author have not always enjoyed acceptance by others. As a result, some lower molar structures have been given as many as five different names (Evander 2004). Also the nomenclature developed for horse teeth does not entirely correspond to the nomenclature of fossil mammals in general. And for Anchitheriine teeth in particular some structures that are described for other fossil horse teeth are not present. Unfortunately, no literature was found describing what the standard Anchitheriine molar structure looks like. But by comparing the structures of the different species of Anchitherium to the nomenclature of fossil horses in general (Evander 2004) it has been possible to deduce at least some of the structures that are missing in, not described for or not used to 32 describe Anchitherium teeth. The following differences in tooth morphology definition are those noted between the nomenclature as proposed by Evander (2004) and the Anchitherium tooth nomenclature as used in Sánchez et al. (1998) and Alberdi et al. (2004); - - - - - - - The minor cusp located posterolabially to the hypocone and anterior to the posterior cingulum was named hypostyle by by Osborn (1918) and Stirton (1941), Sánchez et al. (1998) and Alberdi et al. (2004) also use this term. It has been renamed ‘hypoconule’ by Evander (2004). Likewise, the paraconule (Evander 2004) is still named protoconule (Osborn 1918, Stirton 1941) in the Anchitherium tooth morphology used by Sánchez et al. (1998) and Alberdi et al. (2004). In (figure 3.3) is translated the ‘valle mesial’ and ‘valle distal’ (Spanish) directly to the terminology of Evander’s (2004) ‘post’ and ‘pre-flexid’. Evander states that one of the former names of the ‘Hypoflexid’ (see next comment) was ‘median valley’ (Quinn 1955). It can only be assumed that the English names for ‘valle mesial’ and ‘valle distal’ would subsequently be ‘mesial valley’ and ‘distal valley’. It cannot be determined for sure whether these are also names proposed by Quinn (1955). But when Evander (2004) discusses the previous names of both forms he does not name mesial and distal ‘valley’ as one of them. The Spanish and French terms have therefore been translated directly to Evander’s terminology. It appears as if the hypoflexid (Evander 2004) is, at least by Sánchez et al. (1998) and Alberdi et al. (2004), not used to describe Anchitherium tooth morphology. Which is rather strange since the post and pre- flexid are used for the ‘valleys’ on the lingual side of the lower cheek tooth. The ‘anterostyle’ is most likely referred to by Evander (2004) as ‘Anterior Accessory Rib’ but this is not certain and therefore it has been named anterostyle in (figure 3.3) The terms ‘paraconid’ and ‘metalophid’ appear to be missing in Evander (2004). No synonyms or clues that these are old names have been found. The paraconid is in fact not present in the equine species. However it is named in the literature about Anchitherium. It is possible that this is a leftover from the original paraconid or that it is a new small form of the tooth. In this case it is falsely named paraconid. Inigo (1997) presents a new term ’internal cingulum’, this is the cingulum on the lingual side of the tooth. 33 Nomenclature 2, language Many articles dealing with species specific tooth morphology in Anchiteriine horses have been published in French or Spanish. For this purpose a nomenclature overview of the terminology in Spanish; French and English has been added (figure 3.3) 34 Materials Following is an overview of the research material present from 11 different sites with Anchitheriine remains in Spain (figure 3.4). It will be discussed whether there is secondary literature about the site revealing what species of Anchitherium is present. All reference fauna’s for these different sites has been attempted to gather for this research. Also the Paleobiological database and the NOW database (Fortelius 2003) was consulted for occurrences of Anchitherium or other Perrisodactyl genera. This secondary information was connected to the measurements in order to determine what species is present at which site. For every site there is a detailed description of the different specimens. A species determination will also be attempted using the different characteristics in horse tooth morphology and comparing them to the characteristics as described in the different Anchitherium species descriptions (Appendix A) Munebrega 1 Samples 55-1017 (P2 sin) and 55-1016 (P2 dex) (damaged) – Hypocone and hypostyle are clearly present and well developed and connected to the posteriolingual side of the hypocone in case of heavy wear. The hypostyle is lower than the hypocone and is triangular in shape. The metalophe connects the hypocone to the ectolophe between the paracone and the metacone. A crochet is not present. A basal cingulum is present from the anterior side at the base of the anterostyle along the lingual side of the tooth to the posterior side ending at the posterior side of the metacone. It is weak on the lingual side of the protocone and also on the lingual side of the hypocone. 55-1015 (P2 sin) (strongly damaged on anterostyle) - The description is the same as the P2 sin discussed above. 55-105 (P3 sin) (Hypocone and metacone absent) - The basal cingulum starts at the anterostyle and runs along the anterolingual base of the protocone. It is absent on the lingual side. A crochet is absent. 55-1017 (P3 sin) - The basal cingulum starts at the base of the anterostyle and runs along the anterolingual base of the protocone and is absent on the lingual side. It then runs from the posterior side of the hypocne to the metacone. The hypostyle is triangular and is separate from the posterior cingulum. There is no crochet present. 55-1016 (P3 dex) – The basal cingulum runs from the anterostyle to the anterolingual base of the protocone, it is absent on the entire lingual side and then runs from the posterior side of the hypocone to the metacone. The metalophe does not have a crochet and the hypostyle is strongly connected to the posterior basal cingulum. 35 55-1019 (P3-P4 sin) – Both have a very small style in the preflexid and a very clear basal cingulum. 55-101 (M2 dex) – small style in the postflexid and a clear basal cingulum. 55-1006 (P4 dex) on the base of both the flexid’s the cingulum is incomplete or it has a cingulum like style. Literature and data In the latest NOW database (Fortelius 2003) Anchitherium is not recorded for Munebrega 1. The only perissodactyla present in the datebase from this site is the Rhinocerotidae Hispanotherium matritense. Species- The species Anchitherium castellanum can be ruled out because in this species a basal cingulum is absent in the lower dentition. Anchitherium matritense can also be ruled out on the bases of the crochet that should be present in all upper jaw dentition except the M2. There is a style present in the preflexid of the lower jaw P4, this also rules out Anchitherium procerum. And this dentition does not resemble the dentition of the species identified as Sinohippus sampelayoi. Armantes 1 Samples 41-564 (3* P or M lower jaw sin) – A basal cingulum is only present on the lingual side. All three molars have a style in the flexid. The style is less pronounced in the presumable M2 molar. 41-503 (M2 dex) – The same description as above applies. The style is less pronounced. 41-505 (P1-P2 dex) – The basal cingulum is present on the lingual side of both teeth. The style is very pronounced in P2. 41-500 (M1 dex) – A basal cingulum is present on the lingual side and on the labial side there is a very weak basal cingulum and a style is present (the style almost seems to be part of the cingulum). 41-501, 41-502, 41-504 (M3 dex, M3 sin, M2 dex). In all teeth the paraconid is very pronounced and all have a basal cingulum on the lingual side. The cingulum is absent on the labial side. A style is also present in all teeth. 36 Literature and data In the latest NOW database (Fortelius 2003) Anchitherium is not recorded for Armantes 1. The only perissodactyla present in the datebase from this site are the Rhinocerotidae Alicornops simorrensis and Hispanotherium matritense. Species- The species Anchitherium castellanum can be ruled out because in A. castellanum a basal cingulum is absent in the lower dentition. A style is present in this species’ lower jaw P2, this is also the reason for which Anchitherium castellanum can be ruled out. Anchitherium castellanum has a high frequency of styles in the lower dentition however it is virtually absent in the P2, and this dentition does not resemble the dentition of the species identified as Sinohippus sampelayoi. Arroyo del Val 4 Samples AR4-471 (P2 dex) – A weak cingulum is present on the labial side of the tooth no style is present. AR4-106, AR4-107 (M3 sin, M3 dex) – A basal cingulum is present only on the lingual sied of the tooth. A style is not present and the paraconid is entirely separate. AR4- 105 (P3 dex) – A very pronounced cingulum is present on the lingual side there is no style. AR4-102 (M2 dex) – There is a cingulum on the lingual side and no style. AR-104 (P4 dex, M1 dex) - There is a cingulum on the lingual side of the tooth and there is not style in either of the flexids. Literature and data In the latest NOW database (Fortelius 2003) Anchitherium is not recorded for Arroyo del val. The only perissodactyla present in the database from this site is the Rhinocerotidae Alicornops simorrensis. According to the handwritten cards provided with the Universities fossil material the species of Anchitherium is A. ezquerrae ezquerrae. Species: A cingulum is present in the lower jaw dentition, this rules out Anchitherium castellanum as a possibility. A style is present in the lower jaw P2, this also rules out Anchitherium castellanum. The fact that the style is absent corresponds with the diagnoses of Anchitherium procerum. And this dentition does not resemble the dentition of the species identified as Sinohippus sampelayoi. 37 Arroyo del Val 6 (AR6 25) upper jaw molar (Anchitherium ezquerrae ezquerrae) Literature and data In the latest NOW database (Fortelius 2003) Anchitherium is not recorded for Arroyo del val. The only perissodactyla present in the datebase from this site is the Rhinocerotidae Alicornops simorrensis. Manchones 1 Samples Noted: These samples are very different from the reference teeth used from the species Anchitherium aurelianense. MA1- 121 (P4 sin) – There is no cingulum present of either side of the tooth. The hypocone is connected to the metalophe. The hypostyle is not entirely triangular and is connected to the hypocone. No crochet is present. MA(2)-52 (P3sin) - There is no cingulum present of either side of the tooth. The hypocone is connected to the metalophe. The hypostyle is not entirely triangular and is connected to the hypocone. No crochet is present. MA1-118 (M1 sin) – There is a cingulum present starting at the paraconide and ending at the Metaconide. The style is present, though very slightly. Furthermore a strange M3 molar is present in the material. It has a slightly wrought form towards the posterior of the tooth and it has no cingulum and no style. Literature and data In the latest NOW database (Fortelius 2003) Anchitherium is not recorded for Manchones 1. The only perissodactyla present in the database from this site is the Rhinocerotidae Alicornops simorrensis. Species: A cingulum is present in the lower jaw dentition, this rules out Anchitherium castellanum as a possibility. A slight style is present in the lower jaw M1 which rules out Anchitherium procerum. this dentition does not resemble the dentition of the species identified as Sinohippus sampelayoi. 38 Buñol Samples BU-2 (P1-M3 sin) (lower jaw complete sinistral side) These teeth all have a basal cingulm that runs all around the tooth, except for P1. The paraconide in the M3 molar is not separate as has been observed in other specimens. (Upper jaw (pre-)molar- not specified). A cingulum is present on the lingual side. The hypocone is connected to the metalophe. The hypostyle is triangular and separate from the hypocone. Literature and data According to the notes enclosed with the universities specimen the Anchitherium material found at Buñol is not further specified than the genus level (Anchitherium sp.). Van der Made (1996) generalizes the Anchitherium fossils found at the Buñol site as Anchitherium sp. According to Fortelius (NOW Database July 2003) however some Anchitherium specimen found at the Buñol site belong to the species Anchitherium aurelianense (Fortelius 2003). The primary reference for this fauna (Crusafont and Santonja 1957) also identifies the Anchitheriine species at the Buñol site as A. aurelianense. In their revision of the genus Anchitherium (Sanchez et al. 1998) also identify the fossils found at Buñol as Anchitherium aurelianense aurelianense as does (Forsten 1991). Other perissodactyl species found at Buñol belong to the family Rhinocerotidae; Lartetotherium sensaniense; Prosentorhinus Sp. and Diaceratherium aurelianense. Another perrisodactyl is the Phyllotillon narsicus of the family Chalicotheriidae (Fortelius, NOW 2003). I am inclined to conclude that the Anchitherium specimen from Buñol all belong to the species A. aurelianense because unlike for example the Rhinocerotidae, modern as well as fossil Equidae often show an appearance of only one species per locality (Janis 2008) Exceptions are for example the coexistence of Hipparion and Anchitherium species at some localities (Höck 2009, Salesa 2004) however this coexistence may have been the cause for Anchitherium to decline and disappear completely. Species: A cingulum is present in the lower jaw dentition, this rules out Anchitherium castellanum as a possibility. this dentition does not resemble the dentition of the species identified as Sinohippus sampelayoi. Nombrevilla Samples (P3, P4 dex) – No cingulum is present. The hypocone is connected to the metalophe and the hypostyle is separate and triangular. No crochet is present. (P4, M1, M2 dex) – A cingulum is present on the lingual side and a style is also present. 39 Literature and data According to the universities’ handwritten guideline cards provided with the material the species present in the Nombrevilla fossil finds is Anchitherium sampelayoi. Salesa (2004) provides evidence for his claim that fossils so far identified as Anchitherium sampelayoi should in fact be included in the Asian genus Sinohippus and thus named S. sampelayoi. The primary reference for this fauna is Freudenthal (1966), in this article the equine species found at Nombrevilla is identified as Anchitherium sampelayoi but in the ‘paleobiology database’ this species is also synonym to Sinohippus sampelayoi. This reflects the changing insight on the grouping of this species within a certain genus over time. A problem however arises with the NOW data set, published in 2003 by (Fortelius 2003). According to this data set the two genera of Equidae found at the Nombrevilla site are; Hipparion koeningswaldi and Anchitherium aurelianense. A co-occurence of both these genera has been described for multiple sites and has been generally accepted (Höck 2009, Salesa 2004). However in past fossil assemblages as in modern faunas generally no more than one species of Equidae is found at the same locality, so the co-existence of three different genera (Anchitherium, Hipparion and Sinohippus) would be quite unlikely. A cooccurrence is not impossible but literature presents no further evidence for a co-occurrence of both these species in Nombrevilla. Other perissodactyl species occurring at this site are Diaceratherium aurelianense; Alicornops simorrensis and Lartetotherium sansaniense, all belonging to the Rhinocerotidae family. And Ancylotherium pentilicum of the Chalicotheriidae family (Fortelius 2003). Torralba 1 samples 18-200 (M3) – There is a cingulum on the lingual side and a style is clearly present. The paraconid is separate from the rest of the molar. 18-202 (P2 sin) – The cingulum appears to be present on both sides of the premolar. The hypostyle is triangular and separate. The hypocone and metalophe are connected and no crochet is present. Torralba 3 samples Noted: This is an abnormally large molar. 40 TOR3-5 (P3) - There is a cingulum present on the labial side of the tooth. The hypocone and metalophe are connected . The hypostyle is sort of triangular but had another shape in the middle (circle) and is separate from the hypocone. There is no crochet. Val de Moros 3A Samples VAL3a-500 (P? dex) – A weak cingulum is present on the lingual side. The hypostyle is not entirely triangular and it is separate. No crochet is present and the hypocone is connected to the metalophe. 41 Material – fossil material and casts Two or three photos per location of the most representative samples (figure 3.4) 42 43 44 Scatter-Plots- Species determination For the determination of the species of Anchitherium from the different Spanish localities scatterplots based on tooth measurements will be used. For the determination of the samples on species level the measurements as presented by Inigo (1997), Salesa et al. (2004) and Sanchez et al. (1999) will be used. The findings will be discussed in relation to the information gathered about the species at the sites prior to the measurements (NOW database, Paleobiological database and the reference fauna articles of the different sites). Problems and inconsistencies will also be discussed. Salesa et al. 2004- 45 Munebrega Manchones Nombrevilla Torralba Buñol 40 35 30 25 20 15 25 35 Figure 3.5- Salesa (2004) measurements vs. research measurements 45 The plot on the right (figure 3.5) is from Salesa et al. (2004) and it shows the relationship between length and width of P3-P4 within the different species of Anchitherium. The plot includes American as well as European species, the American species generally being larger. Anchitherium sampelayoi plots high, close to the American species. Salesa et al. use this in their argument that A. sampelayoi ought to be classified under de genus Sinohippus because all the European Anchitherium species plot much lower in this figure. The data from the practical research has been plotted into a similar graph (figure 3.5) in order to identify the different species at the different localities. One problem that directly arises is the fact that Salesa et al. (2004) fails to mention whether for their measurements of P3 and P4 they have used upper or lower jaw molars. It was assumed that they had used the upper jaw molars, and after plotting both figures it became clear that the figure by Salesa et al. (2004) is indeed based on upper jaw molar measurements. The two samples from Nombrevilla are of the newly defined species Sinohippus sampelayoi (Salesa et al., 2004) and might thus form a good calibration for the graph. One value, as measured on my samples plots as S. sampelayoi in Salesa’s figure, the other plots a little too low. Overall Sinohippus sampelayoi appears to be a good match and thus reference point. However, an important thing needs to be noted here; the samples in the universities collection are with a high degree of certainty a cast of the originals that were used by Salesa et al. (2004). The measurements from the practical research and theirs have thus been performed on exactly the same specimens, fossil and cast. Therefore the offset is only explainable by difference in measuring method. I may conclude from this that my method of measuring differs from that of at least Salesa et al. (2004). However the values do plot roughly in the same area so it was concidered the measurements of S. sampelayoi valid. The samples from Munebrega all plot very low, in the area of Salesa’s graph that is only occupied by A. aurelianense. For Munebrega no literature or occurrences was found in the different databases suggesting which species of Anchitherium was present there. It can thus tentatively be concluded (from the very few datapoints ) that the species most likely present at Munebrega is Anchitherium aurelianense. Noted here needs to be that the few specimens that were measured for Munebrega were almost all broken and incomplete. In some cases it was compensated for missing parts by estimating how much of the particular tooth was missing. This was never more that 3 to 4 millimeters. The samples from Buñol also plot in the same area, but lower than Munebrega. For many samples from Buñol it was not specified whether the tooth was a molar or premolar and which one it was. Most of them were eventually classified within the ‘Unidentified Upper jaw molars’ (Appendix B). The values have however been plotted in this graph. The reason why the measurements may plot too low is that the teeth were not P3 and P4 But maybe P2 or M molars. Information from the reference fauna literature and databases shows that the species at Buñol is most likely to be Anchitherium aurelianense as well. The measurements are somewhat consistent with that even if they are slightly too low. The only sample from Torralba plots so far from any of Salesa’s measurements it will not be identified as Anchitherium aurelianense. There was only one other specimen from Torralba 46 but it was concluded that this tooth was so different from the other that it might not even be an Anchitherium molar. Only one measurement remained and it showed such a deviation I ruled it not acceptable as a semi-valid species determination. The only observation that can be validly made is that it plotted by far nearest to A. aurelianense. For Manchones however it’s more complicated. The samples plot generally in the area of A. castellanum, A. matritense and A. alberdiae (and maybe also A. cursor). The fossils have however been determined earlier as belonging to the species A. ezquerrae ezquerrae. This species however has in 1997 by (Inigo 1997) been incorporated into the species Anchitherium corcolense, but only the ones that have been found at Corcoles. It becomes problematic because in Salesa’s figure the species A. corcolense is absent. In the next part Inigo’s figures that do include A. corcolense will be plotted. Here a new hypothesis for the species found at the Manchones site will be presented. Inigo 1997In his presentation of the new species Anchitherium corcolense, Inigo presents a figure in which he plots the values for all different teeth with measurements of A. ezquerrae and A. aurelianense (figure 3.7). He aims to show that A. corcolense is a different species from the A. aurelianense and A. ezquerrae found at different localities. The research data was plotted in a similar fashion (figure 3.6) for comparative purposes. The alternative notation for upper and lower jaw as used by Inigo was maintained only in figure 3.6 for better comparison to figure 3.7. Figure 3.6 – research measurements 47 Figure 3.7 – Inigo (1997) measurements The P1 samples of Buñol plot in the general area of A. aurelianense, as was expected. The P3P4 samples of Buñol plot apparently too low. This has the same cause as the anomaly of these samples within the figures of Salesa. Namely that these molars have not been identified (see appendix B samples are listed under ‘unknown’). Thus the molars from Buñol are probably not P3 or P4 but rather P2 or M1 or M2. The lower jaw M1 data point from Buñol plots nicely within the range of A. aurelianense. The (single) P4 sample however plots inexplicably far outside the area designated for A. aurelianense. I have no explanation for this except that this sample from Buñol perhaps wasn’t a P4 lower jaw molar. 48 The Torralba sample, again, plots so off-chart than I deem it not acceptable as any kind of species determination. The Munebrega P3-P4 samples plot a little outside but still fairly close to the range identified for the species A. aurelianense. This is also consistent with the findings from Salesa’s figure. The P1 sample however plots within the range of A. corcolense which is very strange. I have no explanation for this except that the sample was identified wrongly as a P1 molar. For the lower jaw the Munebrega samples also plot fairly well within the ranges designated for A. aurelianense. Only for the lower jaw M1 it appears as if the samples were too long. Which is possible since identifying the M1 from for example P4 has proven difficult, as confirmed by different authors and by myself. The upper jaw P3-P4 samples for Nombrevilla (L28; W38) and (L30,5; W34) and also the lower jaw M1 sample (L29,5; W21.75) plot outside of the range of the graphs. Which is again consistent with Salesa’s conclusion that these teeth are substantially bigger than the average European Anchitheriinae. Newcomers in this graph are Armantes 1 and Aroyo del Val. From these sites the lower jaw teeth were more abundant in the set I received. Saying something about Aroyo del Val is difficult. The Utrecht University specimens are identified as A. ezquerrae ezquerrae but the lower jaw data plots too low for the ezquerrae samples. In the case of the lower jaw M1 it even appears as if the data point plots more within the area of A. corcolense. It could be hypothesized that the species found at Aroyo del Val may also be in fact A. corcolense. But this will not be done based only on one measurement. This hypothesis will be proposed for the samples from Manchones (next paragraph). The (single) lower jaw M1 Armantes sample plots on the edge of the area designated by Inigo (1997) as A. aurelianense. Again this is too little information to base an actual species determination on. Also because there is no additional information for this site that can confirm that this species is present here. If this additional information were present this single data point may have served as support or confirmation. On its own it is in my opinion rather useless. These plots, based on Inigo (1997), may also shed some light on the problem that arose with Salesa’s figure and the samples from Manchones-1. In the P3-P4 figure, the same proxy that was used by Salesa, the Manchones samples plot well within the range of A. corcolense. They are in the overlapping zone of A. corcolense and A. ezquerrae. According to Inigo only the samples originally classified as A. ezquerrae ezquerrae that where found at the site of Corcoles were to be taken into the new species A. corcolense Inigo, 1997. The fossils from Manchones-1 have been identified as belonging to the species A. ezquerrae ezquerrae by the Utrecht University (date and person unknown). If identified correctly this figure might be a clue that the species found at Manchones-1 might in fact also be A. corcolense, and this then would imply that A. corcolense is not only found at the site of Corcoles. For the lower jaw it is less apparent. In the M1 graph the samples from Manchones plot slightly outside the range of A. corcolense but neither do they plot within the range or any of the other species. So for the M1 figure I would still classify them as possibly A. corcolense. The only possible exception 49 is the plot P4 where it appears as if the (singly) sample from Manchones plots within the range of A. aurelianense. But it plots very near the overlapping zone between A. aurelianense and A. corcolense. And also in the plot P4 the zones of overlap between A. ezquerrae ezquerrae and A. aurelianense with A. corcolense are very close together. This then still leaves intact my theory that possibly A. ezquerrae ezquerrae from Manchones is in fact also A. corcolense. The location of the site Corcoles was plotted in figure (figure 3.8) Both sites are roughly 200 km apart. Thus a co-occurrence of the same species may not be very strange. Figure 3.8 – the location of Corcoles relative to the other locations. 50 Sanchez et al. 1998 Figure 3.9 Sanchéz et al (1998) measurements 51 Sanchéz et al. has named typelocalitiess for his identified new species of Anchitherium. The mean values of the different molars and premolars were plotted and the same was done for the practical research’s measurements. The aim was to be able to match up these measurements with Sanchéz’s and find out whether some of ‘his’ species could be identified in one of ‘the research’ locations. First of all, all of Sanchéz’s species, except for Anchitherium aurelianense that is clearly smaller, group very close together. This already makes a species determination difficult. The only clear match is that of Buñol. This is no surprise since Sanchéz’s data also comes from Buñol. The rest of the data is unfortunately useless. In my opinion Sanchéz’s data is the least reliable. Because he presents no less than 5 new species of Anchitherium that are very much alike. At least their measurements all plot very close together. The fact that the only ‘known’ other species Anchitherum aurelianense is the only one to plot away from the others makes this even more clear. These measurements have been omitted from the final species determination. Species determination - practical summary In this summary of my practical work it will be attempted to determine what species is most likely found at what location. This will be discussed per location separately and will be supported by different measurements and data. Munebrega 1 Based on the morphological research Anchitherium castellanum, Anchitherium matritense, Anchitherium procerum and Sinohippus sampelayoi can be ruled out for Munebrega 1. The NOW database does not have any data on this location. In size comparison to Salesa’s data the samples from Munebrega plot in the area of the graph that is only occupied by the species Anchitherium aurelianense. Also in the data by Inigo the Munebrega samples plot fairly close to the range identified for the species Anchitherium aurelianense for the upper as well as the lower jaw dentition. Judging from these two different data comparisons it would seem as if this species can tentatively be identified as Anchitherium aurelianense. However in the final set of datapoints, from Sanchéz, the Buñol samples (already positively identified as Anchitherium aurelianense) are the only samples plotting near Sanchez’s Buñol samples (in Sanchéz identified as A. aurelianense). The Munebrega samples appear to be much larger. This is strange since in the data by the two other authors both locations appeared to fall well within the range of Anchitherium aurelianense. I have no explanation why in sanchez’s data Buñol does appear to be correct and Munebrega does not. Other than that his data is less reliable than the others (something I am inclined to believe since he miraculously created 5 new species of Anchitherium) and that Buñol is correct by chance where the rest of the data is flawed. The species at the site Munebrega 1, Anchitherium aurelianense 52 Armantes 1 Based on the morphological research Anchitherium castellanum and Sinohippus sampelayoi can be ruled out. The NOW database does not have any data on this location. No samples comparable to Salesa’s data was available for this location. The one datapoint that is comparable to Inigo’s data plots in the range of Anchitherium aurelianense. However this is not enough information to perform a decent species determination. No species determined for this location. Arroyo del val 4 Based on the morphological research Anchitherium castellanum, Anchitherium procerum and Sinohippus sampelayoi can be ruled out. Sánchez et al. (1998) also state that the material from Arroyo del Val could not be assigned to any of the proposed new species and remains Anchitherium sp. The NOW database does not have any data on this location. No samples comparable to Salesa’s data was available for this location. A weak argument may be made for the determination of the species at the location Arroyo del val of Anchitherium corcolense. This would be based on the data presented by Inigo et al. However I will not hypothesize the species Anchitherium corcolense based on only one sample. No species determined for this location. Manchones 1 Based on the morphological research Anchitherium castellanum, Anchitherium procerum and Sinohippus sampelayoi can be ruled out. The NOW database does not have any data on this location. The samples have been earlier identified as A. ezquerrae ezquerrae. This species has by Inigo been incorporated into Anchitherium corcolense nov sp. From Salesa’s data it is hard to say anything because the species Anchitherium corcolense is not present in the data. In Inigo’s data however the data plots in the overlapping zone between A. ezquerrae ezquerrae and Anchitherium corcolense. I find it confusing that Inigo incorporates the (former) species A. ezquerrae ezquerrae as described by Abusch (1983) in his new species. Because if this sample is Anchitherium corcolense this would imply that this species is not only found at the type location but also at the site Manchones 1. Both locations are also only a rough 200 km apart this also makes this hypothesis more likely. The species found at Manchonces 1 may be Anchitherium corcolense. Buñol Based on the morphological research Anchitherium castellanum and Sinohippus sampelayoi can be ruled out. Van der Made (1996) does not further specify the species found at Buñol. In the NOW database by Fortelius (2003) however some specimen are described as Anchitherium aurelianense. Also the primary reference for this fauna Crusafont and Santonja 53 (1957) identifies the species found at Buñol as Anchitheriun aurelianense as do Sanchez et al. (1998) and Forsten (1991). Within the data of Salesa, Inigo and Sanchéz the specimens from Buñol are the only ones always significantly within the range identified as Anchitherium aurelianense. The species at the site Buñol Anchitherium aurelianense. Nombrevilla For the samples from Nombrevilla I can be very brief. The samples in possession of Utrecht University are copies of the exact same specimens that were used as the holotype for the new species Sinohippus sampelajoy. The species found at Nombrevilla is Sinohippus sampelajoy. Torralba 1 and 3 No species determined for this location. Val de Moros 3 A Sánchez et al. (1998) name the species found at the Val de Moros site Anchitherium parequinum. However I could not compare my measurements from Val de Moros to Sánchez’s. Therefore I have not enough information to soundly base a species determination. No species determined for this location. 54 Chapter 5 - Discussion Diversity of species within the Genus Anchitherium The hypotheses to be tested originates from an article by Eronen et al. 2009. They challenge the generally accepted theory that Anchitherium did not adapt to environmental and climatic changes and thus became extinct. They conclude that the Spanish Anchitheriine horses did show signs of ‘incipient hypsodonty’ and thus adaptation to a drier environment. They compare the Spanish species with German Anchitherium species and concludes that these specimens do not show this incipient hypsodonty. They are challenged by Salesa et al. (2010) who point out that Eronen and his group do not take into account the actual diversity of the Iberian representatives of Anchitherium. The German specimens are from a homogeneous selection because they came from a single location. The Spanish specimens were collected over a wide spatial and temporal range. This comment is well grounded and also a point of critique I wish to express in the discussion of this thesis. Aside from the fact whether or not the Spanish Anchitheriine horses did show signs of incipient hypsodonty this research is invalid because the comparison cannot be made on the basis of only one German locality and species. For a better comparison more localities and more species from Germany should be included. A problem of almost all studies used in this thesis that their conclusions about Anchitherium’s adaptation are based on several different species. From the practical research it can be concluded that the diversity of traits and morphological features within the genus Anchitherium is very high. An unfortunate effect of this is that all researches, especially those based on morphological features of a few species can never represent the entire genus of Anchitherium. Morphological changes may have occurred in one species alone. Eronen et al. (2010) defend their methods by stating that looking at the genus level can be a valuable way of gaining information. I disagree with this because when all the species are so morphologically different the genus level will not reflect the overall changes very well. Especially if some remained very conservative whereas other species underwent major change. A discussion point may also be raised about this diversity in species within the genus Anchitherium. The validity of the new species by Sánchez et al. 1998 In their 1998 article Sánchez et al. identify no less than 6 new species within the genus Anchitherium. These species are A. cursor (MN 6 Alhambra-Tüneles, Madrid), A. castellanum (MN 4 La Retama, Cuenca), A. matritense (MN 5 La Fuentecilla, Madrid), A. alberdiae (MN 5 all sites from Madrid), A.procerum (MN 6 Paracuellos, Madrid) and A. parequinum (MN 5 El Terrero and Valdemoros, Zaragoza). The validity of these 6 new is discussed here for several reasons: first of all, all locations except that of the new species A. parequinum, are near Madrid. So basically all species were found geographically close to one another. The different species are however distinctly separated in a temporal range however and span multiple different MN zonations. A second, stronger, argument for the invalidity of (most of) these species is the data that Sánchez et al. present. From the figures on page 47 is becomes 55 clear that the tooth measurements, usually a valuable tool for telling species apart, are useless. All of Sánchez’s measurements on the ‘new’ species dentition plot extremely close to each other. The species are not separable from the other species in none of the plots generated. The only distinct species that can be identified from the figures generated from Sánchez’s data is the species A. aurelianense that clearly has a smaller dentition, which is also recognized by Sánchez et al who base their differential diagnoses mainly on morphological differences in the teeth and bone structure. The differences in especially tooth morphology I judge so minimal that it is not necessarily due to variation between species but possibly variety within one species. The size of the male canines is often named in the differential diagnoses of the new species presented by Sánchez et al. (1998). However this feature can easily be a ‘racial’ difference rather than a morphological difference on the species level. If we look at how different the members of the species Equus ferus caballus Linnaeus, 1758 are it can be concluded that large differences are possible within one equine species. Mainly based on the minimal differences in dental size I would say that most of these 6 new species probably belong to one existing or new species. The only species that might be a genuinely separate species is Anchitherium parequinum. One reason to believe this is the fact that it is the only geographically detached species and the fact that Sánchez et al. (1998) also argue that it is found at Val de Moros. Unfortunately this is the only species for which Sánchez et al. (1998) do not provide dental measurements! If it were a separate species it would be expected to plot away from the other datapoints as Anchitherium aurelianense clearly does. This however remains untestable. These new species as defined by Sánchez et al. (1998) are used by Eronen (2009) in his research on incipient hypsodonty in Spanish and German Anchitherine horses. Eventually Eronen generalizes the Spanish species to genus level and thus it is irrelevant whether these are all separate species or not. Salesa et al. (2009) in his comment on Eronen (2009) acknowledges all of these different Spanish species of Anchitherium. He emphasizes that there are many Spanish species of Anchitherium. If both these scientists acknowledge the validity of all of these species then maybe discarding them on the basis of what was discussed previously may not be the best option. More research is needed and good comparable measurements should be provided for these 6 new species. Spatial and temporal environmental variations in Spain Another major part of this thesis is the role played by climatic and environmental change. An important point on which Eronen et al. (2009) base their assumptions of the adaptation of Spanish Anchitherium is the fact that Spain was the first region to experience aridity during the Early Miocene of Europe. However, from the literature research conducted for this thesis it would almost appear as if various climates and environmental conditions were present at the same time in the same region. Many researchers agree that the overall Miocene climate became cooler and dryer. Böhme et al. (2008) argue 2 ‘washhouse climates’ during the Miocene age took place. The first of these two took place within the timespan of 10.2-9.8 Ma. And Böhme isn’t the only researcher arguing a wet period during MN 9 in Spain. Van Dam (2006) also comes to a similar conclusion. Fortelius et al. (2002) conclude that the Miocene was green with only the slightest hint of increasing aridity, the value of his proxy 56 however is arguable. Other researchers however do support an overall aridification of the interior of the continents and the Iberian Peninsula, like Bruch et al. (2007). The solution is to be found in a phenomenon that Mosbrugger et al. (2005) call ‘local endemism’. Even though for the overall climatic conditions it may be said that they became dryer, in Spain this local endemism was so large that small island environments persevered. This is why many researches appear to contradict each other. The studies were performed in different places and the large regional climatic and environmental differences during the Miocene cause the results of these studies to vary immensely. It is apparently well known that these local differences exist and many authors do note that this is a known factor. Still they base a climatic reconstruction of a large region upon one or two different sites, or many different sites that are located very near each other. One of the goals of this thesis was to map the local climates in Spain during the early late Miocene. It appeared impossible however to link the different species that showed different evolutionary traits to different areas with a certain climate and environment. For the simple reason that both types of studies were not detailed enough. The studies of Anchitherium’s evolutionary adaptations were mainly conducted on genus instead of species level. And the climatic researches about South Western Europe and Spain in general only speak of large local differences. Hypsodonty, the spread of the C4 grasses and Anchitherium’s adaptation Hypsodonty Fortelius et al. (2002) use level of hypsodonty as a proxy for an environmental condition the call ‘generalized water stress’. There is still an ongoing discussion about whether or not hypsodonty is an adaptation to dry food or abrasive food like grass. By calling the condition ‘generalized water stress’, they evade linking hypsodonty to grassland. Apart from this there is also always the possibility of an inmoving already hypsodont species making the area appear much drier. Another point of discussion is why the environmental reconstruction Fortelius presents changes so severely when adding horses to the hypsodont species? His theory works out well when comparing his data to existing climatic records. However hypsodonty in horses seems to disturb the proxy. This is an interesting point of discussion that Fortelius does not elaborate much on. If hypsodonty is a good proxy for aridity, then why don’t horses respond in the same manner as ruminants? Eronen et al. (2009) claim incipient hypsodonty for the Spanish specimens of Anchitherium. Forsten (1991) however states that Anchitherium never became hypsodont. The definition of hypsodont is clearly also a point of discussion. Eronen et al (2009) challenges Forsten’s definition of hypsodont in his article. He states that an increasing steepness of cusp slopes is a prerequisite for truly hypsodont molars. That is why he calls it incipient hypsodont and it is probably why Forsten does not refer to these species as hypsodont. I would also like to question Eronen’s definition of hypsodonty. He does defend that morphological research has shown that increase in cusp slope is one of the first steps towards hypsodonty. But there has been very little actual research in this direction. Is increased cusp slope really a precursor of actual hypsodonty? I find Eronen’s ‘evidence’ not very compelling and suggest that more 57 research needs to be done to find out if increased cusp slope is really an early stage in the development of hypsodonty in horses. Forsten (1991) conciders the species A. aurelianense from Buñol a primeval species and observes an abrupt increase in dental size in the Spanish Anchitheres from the middle Orleanian. Inigo 2001 argues that the species A. corcolense was already separated from the most primitive forms and possessed larger dentition. A. corcolense is slightly older than the specimens from Buñol. Forsten links this increased tooth size to a mixed feeding diet. This may indicate that members of the genus Anchitherium were already showing signs of mixed feeding in the Early Miocene (MN 3) Spread of the C4 grasses There is still much discussion about the exact timing of the spread of the C4 grasses. According to Eronen et al. (2009) the spread of the C4 grasses started with the aridification of the continents during the late Miocene. They contradict themselves here because they also state that the aridification of the continents starts at the beginning of the Miocene. But apparently the environmental consequences were most severe near the early late Miocene. Several other studies place the spread of the C4 grasses later. Janis (1994) places it at around 8 Ma And paleosoil studies by Quade et al. (1992) and Morgan et al. (1994) place the spread of the C4 grasses even later, at the Miocene Pliocene boundary between 7-5 Ma And also Macfadden (1994) concludes from his isotopic values of grazing species that lived during the late Miocene that they ate predominantly C3 grasses so that this was most likely still the dominant type of grass. The hypsodonty discussion is still ongoing, the same goes for the discussion about the spread of the C4 grasses. What is relevant for this thesis is the link to Anchitherium’s extinction. A possible scenario is that Anchitherium witnessed the upcoming of the C4 grasslands that spread at the expense of Anchitherium’s main food source woodland and that hypsodonty was a trait needed to access this new food source. Linking the spread of the C4 grasses to Anchitheriums demise is in this case hard to do. And also linking Anchitherium’s downfall to aridity is risky. Because even though most researchers agree that there was an overall increasing aridity of the interiors of the continents that started on the Iberian Peninsula there is also evidence that there were several wetter periods, one within the range of Anchitheriums extinction period. 58 Chapter 6 - Conclusion The central theme of this thesis is the faunal turnover in the fossil record of the Late Miocene from faunas dominated by Anchitheriine horses with low crowned molar teeth to faunas with Hipparionine horses characterized by high crowned teeth. The general belief is that the spread of the Hipparionine horses as well as the demise of the Anchitheriine horses is associated with the expansion of open habitats and the spread of the C4 type grasses. The Anchitheriine horses and their demise are the central subject of this thesis. And because the environmental change, aridification and increasing openness of the landscape, supposedly started on the Iberian Peninsula especially the Spanish Anchitheriine horses from the Middle to late Miocene are examined both in literature and in practical research. In recent research by Eronen et al. (2009) and Kaiser (2009) evidence is presented that Anchitherium may yet have adapted to the changing environmental conditions of the early late Miocene after all. Eronen et al. compare the tooth morphology of Spanish species of Anchitherium to German species and concludes that the Spanish specimens show signs of incipient hypsodonty and the German specimens do not. This incipient hypsodonty may be indicative of a change in feeding strategy from browsing to mixed feeding. Kaiser however applying the micro and meso-wear proxy on a species of Anchitherium found in Germany, also find his results indicative of a change in feeding strategy from browsing to mixed feeding. The main questions of this thesis were established as follows; - Did Anchitherium adapt to eating grass (mixed feeding) or did Anchitherium fail to adapt to the new environmental conditions? - And, shortly after the Hipparion-date, did Anchitherium go extinct because it didn’t adapt or didn’t adapt quick enough to a changing environment or was it due to competition of the Hipparion horse in its feeding (Kaiser 2009) and or living niche? - Did the environment change so drastically during the early late to late Miocene that Anchitherium was likely to be forced into evolutionary adaptation or face extinction? It was attempted to answer these questions by presenting an literature overview of the researches on these different topics and interlinking them. Unfortunately the practical part of this thesis did not consist of repeating some of the original researches into micro and mesowear of the Anchitherium molars an putting these results into the perspective of these studies as was originally planned. There is no direct answer to the first question. It is however more likely that it was not only the adaptation to eating grass that was important to Anchitherium’s survival. The entire climate changed continuously and the spread of the C4 grasslands cannot even be placed at the exact time of Anchitherium’s extinction. Most researchers place it much later during the Miocene-Pliocene transition. It is very likely that when the climate changed and the 59 environment became more arid that Anchitherium had to change food sources. The evidence also supports this. It is also very likely that Hipparion introduced competition in Anchitherium’s feeding niche. However this may not be only because Anchitherium partly entered Hipparion’s grazing niche, or Hipparion partly entered Anchitherium’s. There is evidence that Hipparion upon entering Europe changed his feeding strategy from grazing to mixed feeding and even browsing. The most likely option for competition is thus that both genera moved towards the same feeding habit of mixed feeding. This adaptation in both genera probably took place before Hipparion migrated into Europe. Changing feeding strategy to a niche that is already occupied by a similar animal does not make sense. A likely explanation for Anchitherium’s demise may be that it was slowly adapting to the changing environment by changing its feeding strategy and just could not handle the pressure of an in migrating similar animal. It is also uncommon for more than one genus of horse to live in the same region the reason for this is probably competition for food resources. This also answers the final question. It does not appear as if the climate changed so drastically that Anchitherium was driven towards extinction. The climate changed constantly and lots of regional differences occurred. But the drastic overturn from woodland to mainly (C4) grassland and savannah that is always pictured as the downfall of the little browsing horse is not supported by most of the literature. Some species of Anchitherium may have adapted more than others showing more signs of hypsodonty or other morphological adaptations. That is one of the main conclusions of this thesis. Researching changes on a genus level seems rather pointless when the differences in species are so extensive. This is an ever returning problem in paleontology. Especially with the larger vertebrates, more species are included in researches to reach sufficient sample sizes for statistical certainty or isotope processing. 60 Short point summary of this thesis - There is a large diversity of (sometimes) contemporary species within the genus Anchitherium therefore looking at adaptations on a genus level seems not very useful. - The validity of the 6 new species within the genus Anchitherium created by Sánchez et al. (1998) is questionable. His claims are based on minimal difference in dental size and also in tooth morphology between the new species (that are almost all from the Madrid basin). For the best morphological distingishable possible new species A. parequinum no measurements are provided by the authors for comparison. - The large spatial and temporal variations (local endemism) in the Spanish Miocene climate and environment make it hard to link them to Anchitherium’s demise alone. - The hypsodonty index as proposed by Fortelius et al. (2002) may be a valuable new proxy for climatic and environmental reconstruction. However the many problems with this proxy that Fortelius brushes away a little too easy do need a lot more research. Is increased cusp slope really an early stage in the development of hypsodonty? And why does the inclusion of hypsodont horse data make the proxy less compatible with climatic reconstructions from the marine records? - The extreme radiation of the C4 grasses most likely took place during the MiocenePliocene boundary (MN 13) and not during the early-late Miocene (MN 9). - The development of hypsodonty is probably not an adaptation to eating grass alone. It is probably an evolutionary trait acquired in many different ways in many different lineages that also appeared to provide an advantage for eating the phytolith rich C3 and C4 grasses. - The reason for Anchitherium’s demise is probably not its failure to adapt to a grazing feeding strategy alone. I was probably both climatic and environmental change combined with an inmigrating very similar animal (Hipparion) occupying more or less the same feeding niche (mixed feeding) that was also better suited for eating grass and drier foliage because it had hypsodont teeth that Anchitherium did not have. Most researchers agree that the climate became drier during the course of the Miocene. 61 References ABUSCH-SIEWERT, S. 1983. Gebiβmorphologische Untersuchungen an eurasiatischen Anchitherien (Equidae, mammalia) unter besonderer Berüksichtigung der Fundstelle Sandelzhausen. In Courier Forschungsinstitut Senckenberg, 62, 1-361 (not seen)* ALBERDI, M. T. GINSBURG, L. RODRÍGUEZ, J. 2004. Anchitherium aurelianense (mammalian, Equidae) (Cuvier, 1825) dans l’Orléanien (Miocéne) de France. In GEODIVERSITAS 26(1), 115- 155 BERNOR, R. L., KOVAR-EDER, J., LIPSCOMB, D. 1988. Systematic, Stratigraphic, and Paleoenvironmental Contexts of First-Appearing Hipparion in the Vienna Basin, Austria. In Journal of Vertebrate Paleontology. Vol 8 (4), 427-452 BRUIJN, DE H. DAAMS, R. DAXNER-HÖCK, G. FAHLBUSCH, V. GINSBURG, L. MEIN, P. MORALES, J. 1992. Report of the RCMNS working Group on fossil mammals, Reisenburg 1990. In Newsletters on Stratigraphy 26(2/3), 65-118 COSTEUR, L., LEGENDRE, S. 2008. Spatial and temporal variation in European Neogene large mammals diversity. In Palaeogeography, Palaeoclimathology, Palaeoecology 261, 127-144 CRUSAFONT PAIRO, M. TRUYOLS SANTONJA, J. 1957. Descubrimiento del primer yacimiento de mamiferos Miocenicos de la Cuenca Valenciana. Notas y Comunicaciones del Instituto Geologico y Minero de Espana 48, 3-22 (not seen) DAAMS, R., VAN DER MEULEN, A. J., ALVAREZ SIERRA, M. A., PELÁEZ-CAMPOMANES, P., KRIJGSMAN, W. 1999. Araonian stratigraphy reconsidered, and a re-evaluation of the middle Miocene mammal biochronology in Europe. In: Earth and Planetary Science Letters, 165, 287-294. DAXNER-HÖCK, G. BERNOR, R. L. 2009. The early Vallesian vertebrates of Atzelsdorf (Late Miocene, Austria) 8. Anchitherium, Suidae and Castoridae (Mammalia). Annalen der Naturhistorischen Museum, Wien 111 A, 557-584 DEMIGUEL, D. AZANZA, B. MORALES, J. (2011) Paleoenvironments and paleoclimate of the Middle Miocene of central Spain: A reconstruction from dental wear of ruminants. In Palaeogeography, Palaeoclimatology, Palaeoecology 302 (34), pp452-463 DOMINGO L., GRIMES S. T., DOMINGO S. M., ALBERDI M. T. 2009 A. Paleoenvironmental conditions in the Spanish Miocene-Pliocene boundary: isotopic analyses of Hipparion dental enamel. In Naturwissenschaften 96, 503-511 62 DOMINGO, D. CUAVAS-GONZÁLEZ, J. GRIMES, S. T. HERNÁNDEZ FERNÁNDEZ, M. LÓPEZMARTÍNEZ, N. 2009 B. Multiproxy reconstruction of the palaeoclimate and palaeoenvironment of the Middle Miocene Somosaguas site (Madrid, Spain) using herbivore dental enamel. In Palaeogeography, Palaeoclimatology, Palaeoecology 272, 53-68 EHLERINGER J. R., MONSON, R. K. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. In Annual Review of Ecological systems 24, 411439 (not seen) EHLERINGER J. R., PEARCY, R. W., 1983. Variation in quantum yield for CO2 uptake among C3 and C4 plants. In Plant physiology 73, 555-559 (not seen) ERONEN, J. T. EVANS, A. R. FORTELIUS, M. JERNVALL, J. 2009. The Impact of Regional Climate on the Evolution of Mammals: A Case Study using Fossil Horses. In The Society for the study of Evolution 64, 2398-408 ERONEN JT, PUOLAMAKI K, LIU L, LINTULAAKSO K, DAMUTH J. 2010. Precipitation and large herbivorous mammals I: estimates from present-day communities. In Evolutionary. Ecological. Res. 12, 217–33 EVANDER, R. L. 2004. (Chapter 16): A Revised Dental Nomenclature for Fossil Horses. In Bulletin of the American Museum of Natural History, Number 285, 209-218 FORSTEN, A. 1991. Size trends in Holarctic Anchitherines (Mammalia, Equidae). In Journal of Paleontology 65(1), 147-159 FORTELIUS, M. SOLOUNAIS, N. 2000. Functional characterization of ungulate molars using the Abrasion-Attrition wear gradient: a new method for reconstructing paleodiets. In American museum novitiates 3301, 1-36. (not seen) FORTELIUS, M. (coordinator) (database July 2003). Neogene of the Old World Database of Fossil Mammals (NOW). University of Helsinki. http://www.helsinki.fi/science/now/. (April 2011) FREUDENTHAL, M. 1966. On the mammalian fauna of the Hipparion-Beds in the CalatayudTeruel basin (Prov. Zaragoza, Spain) Part I. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. Series B: Palaeontology, Geology, Physics, Chemistry, Anthropology 67(5), 296-317 (not seen) GOULD, S. J. VRBA, E. S. 1981. Exaptation – a missing term in the science of form. In Paleobiology 8, 4-15 GRAY, J. E. 1821 – On the natural arrangement of vertebrose animals. London Med Reposit. Rev. 15, 296-310 (not seen)* 63 HAYEK, L. A.C., BERNOR, R.L, SOLOUNIAS, N., STEIGERWALD P. 1991. Preliminary studies of Hipparione horse diet as measured by tooth microwear. In: Bjorn Kurten. A Memorial Volume. Annales Zoologici Fennici. 28(3-4), 187-200 (not seen) HERNÁNDEZ FERNÁNDEZ, M. SALESA, M. J. SÁNCHEZ, I. M. MORALES, J. 2003. Paleoecology of the genus Anchitherium von Meyer, 1834 (Equidae, Perrisodactyla, Mammalia) in Spain: evidence from macromammal faunas (original title; Paleoecología del género Anchitherium vonr Meyer, 1834 (Equidae, Perrisodactyla, Mammalia) en España: evidencias a partir de las faunas de macromamíferos). In Coloquis de Paleontologica, Vol. Ext. 1, 253-280 HORDIJK, K. 2010. Perseverance of Pikas in the Miocene. In: Geologica Ultraiectina, Mededelingen van de Faculteit Geowetenschappen departement Aardwetenschappen Universiteit Utrecht No. 333 (Phd thesis) INIGO, C. 1993. Estudio de los Perisodáctilos del yacinmiento Mioceno de Córcoles (Guadalajara). Thesis Doctoral Facultad de Ciencias Biológicas de Universidad Complutense Madrid; 559 p. Unpublished (source: Inigo 1997) INIGO, C. 1997. Anchitherium Corcolense Nov. Sp., a new Anchitherine (Equidae, mammalian) from the early Aragonian site of Córcoles (Guadalajare, Spain). In GEOBIOS, 30, 6: 849-869 JANIS, C. 1995. An Evolutionary History of Browsing and Grazing Ungulates. (chapter) In The Ecology of Browsing and Grazing Ecological Studies 195 (Gordon, I. J. Prins, H.H.T.) 21-45 © Springer 2008 (and published) In Developments in Palaeontology and Stratigraphy Volume 14, 1995, Pages 147-189 JIMÉNEZ-MORENO, G., FAUQUETTE, S., SUE, J. P., AZIZ, H. A. 2007. Early Miocene repetitive vegetation and climatic changes in the lacustrine deposists of the Rubielos de Mora Basin (Teruel, NE Spain). In Palaeogeography, Palaeoclimatology, Palaeoeocology. 250, 101-13 (not seen) KAISER, T. M., SOLOUNIAS, N., FORTELIUS, M., BERNOR, R. L., SCHRENK, F. 2000. Extending the tooth mesowear method to extinct and extant equids. In: Geodiversitas 25(2), 321-345 KAISER, T. M., SOLOUNIAS, N. 2003. Extending the tooth mesowear method to extinct and extant equids. In Geodiversitas 25(2), 321-345 KAISER, T. M. 2009. Anchitherium aurelianense (Equidae, Mammalia): a brachydont “dirty browser” in the community of herbivorous large mammals from Sandelzhausen (Miocene, Germany). In Paläontologische Zeitschrift 83, 131-140 64 LEIDY, J. 1869. The extinct mammalian fauna of Dakota and Nebraska: Including an account of some allied forms from other localities, together with a synopsis of the mammalian remains of North America. Published for the Academy by J.B. Lippincott, Philadelphia* LINNAEUS, C. 1758. Systema naturae per regna tria naturae: secundum classes ordines, genera, species, cum charateribus, differentiis, synonymis, locis. In Holmiae (Laurentii Salvii). P. 73* MACFADDEN, B. J. CERLING, T. E. 1994. Fossil horses, carbon isotopes and global change. In Elsevier Science, TREE vol. 9, no 12pp 481-485 MACFADDEN, B. J., CERLING, T. E. 1996. Mammalian herbivore communities, ancient feeding evology, and carbon isotopes: a 10 Myr sequence from the Neogene of Florida. In: Journal of Vertabrate Palaeontology 16(1), 103-115. (not seen) MADE, VAN DER J. BELINCHÓN, M. MONTOYA, P. 1996. Suoidea (mammalia) from the Lower Miocence Locality of Buñol, Valencia, Spain. GEOBIOS, 31, 1:99-112 MEYER VON, H. 1844. Die fossilen Knochen aus dem Tertiär-Gebilde des Cerro de Sand Isidro bei Madrid. In Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 289-310 (not seen)* MOLNAR, P., 2005. Mio-Pliocene Growth of the Tibetan Plateu and Evolution of the East Asian climate. In Palaeontologia Electronica 8 (1), 1-23. MORGAN, M. E., KINGSTON J. D., MARINO B. D. 1994. Carbon isotopic evidence for the emergence of C4 plants in the Neogene from Pakistan and Kenya. In Nature 367, 162-165 MOSBRUGGER, V., UTESCHER, T., DILCHER, D. L. 2005. Cenozoic continental climatic evolution of Central Europe. In Proceedings of the North American Science Society Vol 102 No. 42 OSBORN, H. F. 1918. Equidae of the Oligocene, Miocene, and Pliocene of North American, inconographic type revision. In Memoirs of the American Museum of Natural History, ser. 2, 1-217 (not seen). PAGANI, M., ARTUR M. A., FREEMAN, K. H. 1999. Late Miocene atmospheric CO2 concentrations and the expansion of the C4 grass. In Science 285, 876-878 QUADE J. CERLING T. E., BARRY, J. C., MORGAN, M. E., PILBEAM D.R., CHIVAS A. R., LEETHORP J. A., VAN DER MERWE N. J. 1992. A 16-Ma record of paleodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. In Chemical Geology 94, 183-192 (not seen) 65 QUADE, J., SOLOUNIAS, N. 1994. Stable isotopic evidence from paleosol carbonates and fossil teethe in Greece for forest or woodlands over the past 11 Ma In: Palaeooceanography, palaeoclimathology, Paleaeoecology. 108, 41-53 (not seen) RETALLACK, G. J. 1983. Late Eocene and Oligocene paleosols from Badlands national Park, South Dakota. In Geological Society of America Special Papers 193, 1-82 RETALLACK, G. J. 2001. Cenozoic expansion of grassland and climate cooling. In Journal of Geology 109, 407-426 RÖGL, F. 1999 Mediterranean and Paratethys Palaeogeography during the Oligocene and Miocene. In: AGUSTI, J., ROOK, L., ANDREWS, P. Hominoid evolution and climatic change in Europe. The evolution of neogene terrestrial ecosystem in Europe, vol. 1 Cambridge Universiy Press, pp. 8-22 (not seen) RÖGL, F., DAXNER-HÖCK, G. 1996. Late Miocene Parathetys Correlations. In The Evolution of Western Eurasian Neogene Mammal Faunas, Wiley, New York pp. 47-55 M. J. SALESA, I.M. SANCHÉZ, J. MORALES. 2004. Presence of the Asian horse Sinohippus in the Miocene of Europe. Acta Palaeontologica Polonica 49(2), 189-196* SÁNCHEZ, I. M. SALESA, M. J. MORALES, J. 1998. Revision sistematica del genero Anchitherium Meyer 1834 (Equidae; perissodactyla) en España. In Estudios geol, 54, 39-63 STIRTON, R. A. 1941. Development of characters in horse teeth and the dental nomenclature. In Journal of Mammalogy 22, 434-446 (not seen) STRÖMBERG, C. A. E. 2005. Evolution of hypsodonty in equids: testing a hypothesis of adaptation. In Paleobiology, 32(2), pp. 236-258 TÜTKEN, T., VENNEMANN, T., 2009. Stable isotope ecology of Miocene large mammals from Sandelzhausen. Southern Germany. In Fossil lagerstätte Sandelzhausen, fauna. Paläontologische Zeitschrift 83(1) (not seen) VAN DAM, J. A. 2006. Geographic and temporal patterns in the late Neogene (12-3 Ma) aridification of Europe: the use of small mammals as paleoprecipitation proxies. In Palaeogeograpy Palaeoclimatology Palaeoecology 238, 190-218 VILLALTA, J. F. CRUSAFONT-PIARO, M. 1945. Un Anchitherium en el Pontiense espanol. Anchitherium sampelayoi, nova sp. In Notas y Communicationes del Instituto Geológico y Minero de Espana, 14, 51-82 (not seen)* 66 VISLOBOKOVA, I., A. 2006. Associations of Ruminants in Miocene Ecosystems of Eastern Alpine Region. In Paleontological journal vol. 40, No 4, pp. 438-447 WILLIAMS, L.H., OWEN-SMITH, N. 2000. The vegetation of the South African Lombard Reserve and its utilization by certain antelope. In Zoologica Africana 1, 55-71 (not seen) QUINN, J. H. 1955. Miocene Equidae from the Texas Gulf Coastal Plain. In University of Texas Publication Bureau of Economic Geology 5516, 1-102 (not seen) ZACHOS, J., PAGANI, M., SLOAN, L., THOMAS, E., BILLUPS, K. 2001. Cenozoic Global Deep-Sea Stable Isotope Data. In Science 292, 686-693 *Taxonomic reference 67