pubdoc_4_1313_1879
advertisement

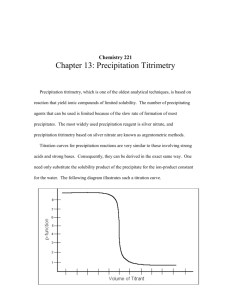
University of Babylon College of Engineering / Al-Musayab Chemistry Manual / First stage PREPARED BY Dr. Ali Jassim Al-Zuhairi M.sc. Mohammed Thamer Al-Zubaidi 3- Precipitation titration Experiment No. (4) Preparation and standardization of AgNO3 solution by Mohr's method Theory:The precipitation method is based on titration with the use of reactions accompanied by formation of sparingly soluble compounds. The most important methods are those based on precipitation of insoluble silver salts in accordance with the equation Ag+ + X AgX The method is based on titration of a halide, such as NaCl with AgNO3 solutions in the presence of K2CrO4 as indicator. The end point of the titration is the point at which the color of suspension changes from pure yellow (due to the presence of CrO42- ions in solution) to reddish brown of AgCrO4. While AgNO3 can be used (p.s.s), but is quite expensive and used after standardization against pure dry NaCl, not KCl because of the traces of water locked in crystals. Notes:1- Solution of AgNO3 should in a dark colored bottle to prevent photo chemical reduction to silver metal Ag+ + e- hʋ Ag 2- In Mohar method Cl- or Br- ion is titrated with Ag+ ion using K2CrO4 as an indicator. Ag+ + Cl- AgCl White p.p.t Ksp= 1.2 ×1010- University of Babylon College of Engineering / Al-Musayab Chemistry Manual / First stage 2Ag+ + CrO- Then At end point While PREPARED BY Dr. Ali Jassim Al-Zuhairi M.sc. Mohammed Thamer Al-Zubaidi Ag2CrO4 Ksp =1.7 ×1010- Ksp (AgCl) = [Ag+][Cl-] = 1.82×1010[Ag+] = [Cl-] = 1.82×1010- = 1.35×105-M Ksp (Ag2CrO4) = [Ag+] 2[CrO4-2]= 1.2×1012[CrO4-2]=(1.1×1012- )/(1.35×105-)= 6×103-M If CrO42- concentration is kept at (6×103-M) the red (p.s.s) of Ag2CrO4 will appear at the end point. Conditions for Mohr's method. 1- The Mohr's method is used for determination of silver, chloride and bromide (it cannot use for iodides and thiocyanates because the results are greatly distorted by adsorption effect. 2- The Mohr's method is suitable in neutral or weakly alkaline solutions (PH = 6.5-10) because Ag2CrO4 is soluble in acids. 3- Absence of cations which from precipitates with CrO42- (such as Pb2+ Ba2+) and anions forming slightly soluble silver salts such as PO43,AsO4,SO32-,S2-,CO32-,C2O42- in Mohr's method. 4- Avoid the formation of Ag2CrO4 by local excess of the titrant before the end point, by shaking the flask. 5- Pure NH4Cl can be titrated by Mohr's method providing that PH lives between (6.5-7.2), at higher PH, too much NH3 is evolved increasing the solubility of AgCl and Ag2CrO4. 6- In concentrated solutions of Ca2+ and Mg2+, the end point appears too soon, ant not sharp, addition of (5-10ml) of (1%) agar solution prevents the agglomeration of the precipitate. 7- Mohr's method can not be used for chlorides of cations which are hydrolyzed such as Al3+, Fe3+,Bi3+, Sn2-, In2+,.......... . University of Babylon College of Engineering / Al-Musayab Chemistry Manual / First stage PREPARED BY Dr. Ali Jassim Al-Zuhairi M.sc. Mohammed Thamer Al-Zubaidi Procedure:standardization of AgNO3:1. 2. 3. 4. Put (5ml) from standard (0.1N) NaCl solution in to a conical flask. Add (1ml) of the chromate indicator solution. Titrate with silver nitrate solution until the color change to reddish. Calculate the molars of AgNO3. Questions:1- What is the principle of titration of bromide and chloride with AgNO3 solution without indicators ? 2- How does K2CrO4 act an indicator in titration of chlorides with AgNO3 solution ? 3- State the conditions for application of the Mohr's method ? 4- State the blank indicator ? 5- Why the solution of AgNO3 is stored in a dark colored bottle ? 6- Why Mohr's method is take place in neutral or weakly alkaline solution ? University of Babylon College of Engineering / Al-Musayab Chemistry Manual / First stage PREPARED BY Dr. Ali Jassim Al-Zuhairi M.sc. Mohammed Thamer Al-Zubaidi Experiment No.(5) Determination of chlorides in presence of Adsorption indicator (Fajan's method). Theory:An adsorption indicator is an organic compound that is adsorbed on or desorbed from the surface of the solid formed during a precipitation titration . Titration involving adsorption indicators are rapid, a curate and reliable. Condition of adsorption indicator:1- The precipitate must be colloidal to maximize the quantity of indicator adsorbed. 2- The (p.p.t) must adsorb it's own ions strongly. 3- The indicator dye must be primary adsorbed ion. 4- The PH of the solution must be such as to assume that the ionic form of the indicator predominates. Examples on adsorption indicator:(a) fluorescein. (b) dichloro fluorescein In this exp. we use fluorescein as adsorption indicator, it is very weak acid, it is used in PH (7-10). University of Babylon College of Engineering / Al-Musayab Chemistry Manual / First stage PREPARED BY Dr. Ali Jassim Al-Zuhairi M.sc. Mohammed Thamer Al-Zubaidi Questions:1. What's mean of adsorption indicator? 2. Write the structure of fluorescein? 3. In which PH the adsorption indicator fluorscein work? 4. What is the condition of adsorption indicator?