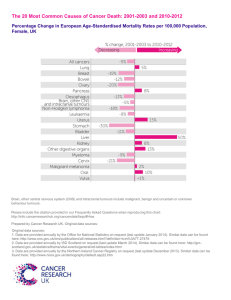
TUmour of the skin and subcutis
advertisement

TUMOUR OF THE SKIN AND SUBCUTIS Laurent Findji DMV, MS, MRCVS, Diplomate ECVS VRCC Veterinary Referrals, Essex, United Kingdom Tumours of the skin and subcutaneous tissues are the most common in dogs, in which it accounts for one third of all tumours, and the second most common in cats (1/4 of all tumours) after lymphoid tumours. By order of decreasing frequency, cutaneous tumours of dogs include mast cell tumours (19%), hepatoid adenoma and adenocarcinoma (10%), lipoma (7%), sebaceous hyperplasia and adenoma (7%), histiocytoma (7%), squamous cell carcinoma (6%), melanoma (6%), fibrosarcoma (6%), basal cell tumours (5%) and hemangiopericytoma/nerve sheath tumours (4%)1. In cats, the 5 most frequently encountered cutaneous tumours are basal cell tumours (20%), mast cell tumours (17%), fibrosarcomas (17%), squamous cell carcinomas (11%) and sebaceous hyperplasia and adenomas (3%) 1. For most of these tumours, the mainstay of treatment is complete surgical excision. For the surgeon, it often comes down to the ability to reconstruct the resulting defect. Reconstructive surgery therefore is in many cases the most challenging part of the surgery. The exhaustive review of these tumours types and behaviour is beyond the scope of this text. Notions of reconstructive surgery in oncology will be discussed elsewhere in this seminar. The particular cases of feline injection-site sarcomas and canine cutaneous and subcutaneous mast cell tumours will be presented. Feline injection-site sarcomas Feline injection-site sarcomas (FISS) include different types of tumours of similar clinical presentation and behaviour, occurring in the location of a previous inflammation (injection, insect bite wound, etc.). They are extremely locally invasive tumours which tend to metastasise infrequently (up to 26%2) and late in their course. However, as veterinarians’ ability to achieve local control of these tumours improve (better planned surgeries, adjuvant radiotherapy, chemotherapy), cats survive longer and it seems that metastases are more frequent a problem than originally reported. In any event, when metastatic disease is not detected at the time of diagnosis, these tumours are mainly a local problem for which an aggressive (wide) resection, whenever possible, is the treatment of choice. In cats, sarcomas developing in the location of an inflammation (“FISS”) tend to be more aggressive than other soft tissue sarcomas3. Practically, sarcomas occurring in areas of injections (vaccine or others) or in the location of an earlier trauma (bite, wound, etc.) must be treated aggressively (wide surgical excision and radiotherapy as possible). When to operate? Any mass located in the location of an injection or inflammation, with clinical and cytological presentations compatible with a sarcoma must be excised widely. How to manage an inflammatory or post-injection reaction is more controversial. When does an inflammatory reaction become suspicious enough to be treated as a malignancy? Most authors base their approach on the guidelines of the Vaccine-Associated Feline Sarcoma Task Force (VAFSTF) which recommends to treat as FISS any inflammatory reaction persisting more than 3 months after an injection, measuring more than 2 cm or still increasing in size more than 1 month after the injection 4. L. Findji – Tumours of the s kin and s ubcutis – AMVAC 2 013 1/5 How to plan surgery? Advanced imaging techniques (contrast-enhanced CT and MRI) are essential to precisely determine the tumour invasion in 3 dimensions, which will dictate the extent of the required surgical excision. The external physical presentation of the tumour can be deceiving and the tumoral volume as determined from a CT study was found to be twice as large as the volume estimated from direct physical measurement of the tumour 5. How to operate? Initially, it has been recommended to excise FISS with a minimum of 2-cm lateral margins and one deep fascial plane. These recommendations are not sufficient and lead to complete excision of such tumours in less than 50% of cases6, 7, which results in high recurrence rates. A more aggressive surgery is associated with better results: in a study of 61 cases, the time to first recurrence was significantly shorter after marginal resection than after wide resection (66 versus 419 days) 6. Similarly, cats operated by non-specialist veterinarians appeared to have earlier recurrences than those operated in a referral centre (66 versus 274 days) 6. Whereas surgery as sole treatment is not currently the gold standard of FISS treatment (combining surgery with radiotherapy and chemotherapy improve the prognosis8), it clearly appears that a more aggressive surgery is associated with a better outcome in terms of disease-free interval and survival times, whether the surgery be used alone or combined with other treatments. Some surgeons recommend excising FISS with 5-cm lateral margins and two deep fascial planes 9. This more aggressive approach resulted in complete excision of the tumour in 97% of cases, leading to a recurrence rate of 11% and disease-free rates of 91%, 86% and 74% at 1, 2 and 3 years, respectively 10. A larger retrospective study of this approach (91 cases) reported a recurrence and metastatic rates of 14% and 20% respectively11. Median survival times were 499 days for cats with tumour recurrence versus 1461 days for cats without11. Median survival times were 388 days for cats which developed metastatic disease and 1528 days for cats who did not11. The drawbacks of such an approach are the more challenging reconstructions and the increased rate of wound dehiscence. In this latter study, 7 out of 91 cats (8%) had dehiscence of their surgical wound11. The wide excision of FISS often involves extensive resections, frequently including bony resections (spinous processes, scapulae, etc.). Similarly, wound reconstruction can be technically challenging and necessitate placement of prostheses, such as meshes, and skin or myocutaneous flaps12. These aggressive resections are very traumatic and induce severe pain. In addition to usual analgesia routes, the use of wound catheters is very efficacious. These catheters are commercially available, but can alternatively be designed from polyethylene tubing2. They are placed in the wound during surgery and are used postoperatively to instil local anaesthetics in the wound for several days after surgery. I routinely use these catheters after FISS resection. They subjectively are very helpful to control postoperative pain and make it possible to lower the doses and limit the side-effects of other analgesic drugs. Canine mast cell tumours of the skin and subcutis Mast cell tumours (MCTs) are complex tumours. Classically, pathologists recognise three grades (1 or low-, 2 or intermediate-, and 3 or high-grade) of increasing malignancy according to Patnaik’s classification. This classification remains an important prognostic factor 13. Unfortunately, the determination of the grade of a MCT is somewhat subjective: a study showed a significant variability in the grade attributed to the same tumour by different histopathologists14. Other classifications have recently been advocated, which begin to be more widely used but remain less common15. Grade-2 MCTs in the classification of Patnaik are by far the most frequently encountered. These MCTs are of highly variable aggressiveness. Additional indicators of their malignancy are therefore required to discriminate between aggressive grade-2 MCTs from more indolent ones (mitotic index, Ki-67, c-KIT, AgNOR, etc.)16 17. In addition, it seems that for a given grade, MCTs are of variable aggressiveness depending on the breed of the affected animal and the location of the tumour. For instance, Boxers are predisposed to MCTs but are frequently L. Findji – Tumours of the s kin and s ubcutis – AMVAC 2 013 2 /5 affected by little-aggressive ones. On the contrary, MCTs of Bernese Mountain dogs, Shar Peis and BullMastiffs are frequently of higher malignancy18. In addition, in all breeds, auricular, ungueal, perianal and oral MCTs, as well as those located on cutaneomucous junctions, are associated with a poorer prognosis 18. Lastly, it is important to appreciate that the presence of multiple MCTs on the same animal, either simultaneously or successively, does not result from the spreading of a single MCT but are distinct and independent diseases. One study did not found any difference in prognosis between animals with single MCTs and those with multiple cutaneous MCTs19. The prognosis associated with canine mutliple MCTs remains good, provided all instances of the disease are treated adequately20. All these considerations are important to determine the appropriate « dosage » of to administer and complete surgical excision, when possible, remains the best treatment for non-metastasised grade 1 and grade 2 MCTs21. The quality of surgical excision (complete or incomplete) has been repeatedly been found to be a major prognostic factor19, 20. When the MCT is located in a area which precludes taking the necessary margins, its excision should be as wide as possible and combination of surgery with adjuvant treatments (radiotherapy, chemotherapy) is indicated. General considerations Mast cell tumours must be manipulated as little as possible, as any manipulation (e.g. pre-surgical scrubbing) can trigger their degranulation. When MCTs are large or seemingly inflammatory before surgery, the administration of anti-histamine or corticosteroids is indicated preoperatively. Surgical excision of cutaneous and subcutaneous MCTs must abide to the general rules of oncologic surgery (see elsewhere in this seminar). In particular, each time that the knowledge of the grade of the MCT will have a significant influence of its surgical treatment, it needs to be graded before surgery by the performance of an incisional biopsy. Lack of planification of the surgery is a major reason for failure of surgical treatment. In particular, any contamination of distant sites with tumour cells when the status of achieved margins (i.e. free of tumour cells, or not) is unknown and advanced reconstruction is necessary must be avoided. Changing gloves, drapes and instruments is therefore frequently required during surgical treatment of MCTs of large size or high grade. Lastly, the following recommendations pertaining to the recommended margins for excision of MCTs are the most widespread in the veterinary literature. They must however not be followed rigidly and blindly because they do not take into account the size of the tumour to excise, which is known to have an impact on the marges required to achieve its complete excision22: the larger the tumur, the larger the margins required. Grade 1 mast cell tumours One-to-2 cm lateral margins and a deep muscular or fascial plane are sufficient 23, 24. Grade 2 mast cell tumours In one study, 75% of grade II MCTs appeared to be fully excised when 1-cm lateral margins were sought and 100% when 2-cm lateral margins were sought24. In all cases, a deep muscular or fascial plane must be excised en-bloc with the tumour. In a more recent study, clusters of mastocytes were present at the resection margins in 2 out of 19 cases of grade II MCTs resected with 2-cm lateral margins23. Therefore, it is advised to seek 3-cm rather than 2-cm lateral margins whenever possible. When it is not possible, seeking 2-cm lateral margins should however lead to complete excision of the tumour in the majority of cases. L. Findji – Tumours of the s kin and s ubcutis – AMVAC 2 013 3/5 Grade 3 MCTs Grade 3 MCTs are consistently aggressive and very frequently metastasise. However, the local control of the tumour, and thereby its complete excision, is important and is associated with a better prognosis than when the tumour is incompletely excised25. For excision of grade 3 MCTs, 3-to-5-cm lateral margins and one or two deep muscular of fascial layers are recommended. Références 1. Vail DM, Withrow SJ. Tumors of the skin and subcutaneous tissues. In: Withrow SJ, Vail DM(eds): Small animal clinical oncology. St. Louis, Mo.: Saunders Elsevier, 2007;375-401. 2. Davis KM, Hardie EM, Lascelles BDX, Hansen B. Feline fibrosarcoma: perioperative management. Compendium Continuing Education for Veterinarian. 2007;29: 712-729. 3. Hauck M. Feline injection site sarcomas. Veterinary Clinics of North America, Small Animal Practice. 2003;33: 553-571. 4. Morrison WB, Starr RM. Vaccine-associated feline sarcomas. Journal of the American Veterinary Medical Association. 2001;218: 697-702. 5. McEntee MC, Page RL. Feline vaccine-associated sarcomas. Journal of Veterinary Internal Medicine. 2001;15: 176-182. 6. Hershey AE, Sorenmo KU, Hendrick MJ, Shofer FS, Vail DM. Prognosis for presumed feline vaccineassociated sarcoma after excision: 61 cases (1986-1996). Journal of the American Veterinary Medical Association. 2000;216: 58-61. 7. Davidson EB, Gregory CR, Kass PH. Surgical excision of soft tissue fibrosarcomas in cats. Veterinary Surgery. 1997;26: 265-269. 8. Séguin B. Feline injection site sarcomas. Veterinary Clinics of North America, Small Animal Practice. 2002;32: 983-995. 9. Kuntz CA. Modified wide local excision for vaccine associated soft tissue sarcomas in cats (abstract). Veterinary Surgery. 2000;29. 10. Liptak JM, Forrest LJ. Soft tissue sarcomas. In: Withrow SJ, Vail DM(eds): Withrow & MacEwen's small animal clinical oncology. St. Louis, Mo.: Saunders Elsevier, 2007;425-454. 11. Phelps HA, Kuntz CA, Milner RJ, Powers BE, Bacon NJ. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998-2002). Journal of the American Veterinary Medical Association. 2011;239: 97-106. 12. Lidbetter DA, Williams FA, Jr., Krahwinkel DJ, Adams WH. Radical lateral body-wall resection for fibrosarcoma with reconstruction using polypropylene mesh and a caudal superficial epigastric axial pattern flap: a prospective clinical study of the technique and results in 6 cats. Veterinary Surgery. 2002;31: 57-64. 13. Thamm DH, Vail DM. Mast cell tumours. In: Withrow SJ, Vail DM(eds): Small animal clinical oncology. St Louis: Saunders Elsevier, 2007;402-424. 14. Northrup NC, Howerth EW, Harmon BG, Brown CA, Carmicheal KP, Garcia AP, et al. Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. Journal of Veterinary Diagnostic Investigation. 2005;17: 561-564. 15. Kiupel M, Webster JD, Bailey KL, Best S, DeLay J, Detrisac CJ, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Veterinary Pathology. 2011;48: 147-155. 16. Bergman PJ, Craft DM, Newman SJ, Baer K, Camps-Palau MA, McKnight JA, et al. Correlation of histologic grading of canine mast cell tumours with Ki67/PCNA/AgNOR/c-Kit scores: 38 cases (2002-2003). Vet Comp Oncol. 2004;2: 98. 17. Romansik EM, Reilly CM, Kass PH, Moore PF, London CA. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Veterinary Pathology. 2007;44: 335-341. 18. North SM, Banks TA. Mast cell tumours. In: North SM, Banks TA(eds): Small animal oncology: an introduction. Edinburgh: Saunders Elsevier, 2009;183-196. 19. Murphy S, Sparkes AH, Blunden AS, Brearley MJ, Smith KC. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Veterinary Record. 2006;158: 287-291. 20. Mullins MN, Dernell WS, Withrow SJ, Ehrhart EJ, Thamm DH, Lana SE. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without L. Findji – Tumours of the s kin and s ubcutis – AMVAC 2 013 4/5 adjuvant treatment: 54 cases (1998-2004). Journal of the American Veterinary Medical Association. 2006;228: 91-95. 21. Séguin B, Leibman NF, Bregazzi VS, Ogilvie GK, Powers BE, Dernell WS, et al. Clinical outcome of dogs with grade-II mast cell tumors treated with surgery alone: 55 cases (1996-1999). Journal of the American Veterinary Medical Association. 2001;218: 1120-1123. 22. Liptak J. The Principles of Surgical Oncology: Surgery and Multimodality Therapy. Compendium Continuing Education for Veterinarians. 2009;31: 14 p. 23. Fulcher RP, Ludwig LL, Bergman PJ, Newman SJ, Simpson AM, Patnaik AK. Evaluation of a twocentimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. Journal of the American Veterinary Medical Association. 2006;228: 210-215. 24. Simpson AM, Ludwig LL, Newman SJ, Bergman PJ, Hottinger HA, Patnaik AK. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumours in dogs. Journal of the American Veterinary Medical Association. 2004;224: 236-240. 25. Hume CT, Kiupel M, Rigatti L, Shofer FS, Skorupski KA, Sorenmo KU. Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997-2007). J Am Anim Hosp Assoc. 2011;47: 37-44. L. Findji – Tumours of the s kin and s ubcutis – AMVAC 2 013 5 /5