Open Access version via Utrecht University Repository

Cryptosporidiosis in pre-weaned calves at the wildlife/livestock interface of the Kruger
National Park, South Africa
Prefactory note
This research is part of the PHD-project, done by Nada Abu Samra at the University of
Pretoria, Onderstepoort, in South Africa. The PHD-project is about Cryptosporidium spp . in livestock, human and wildlife at the interface of the Kruger National Park (KNP).
Abstract
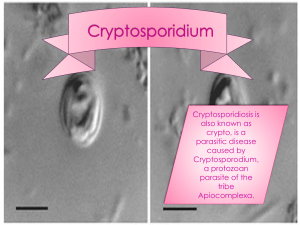
Cryptosporidium is a protozoa, which affects animals and human worldwide. This project is part of a broader study looking at the zoonotic aspect of Cryptosporidium spp. at the wildlife/livestock and human interface of the KNP. The purpose of this study is to detect the prevalence and risk factors of cryptosporidiosis for calves living in a rural farming area close to the KNP. Therefore we collected 354 fecal samples of individual calves in the Mnisi
Traditional Authority. Together with every sample we have done a survey with questions focused on possible risk factors for the infection of Cryptosporidium . All the samples were analyzed microscopically using a Modified Ziehl Neelsen staining method and were viewed under the microscope for Cryptosporidium oocysts. In this study only 2 positives were detected out of the 354 fecal samples. Resulting in a prevalence of 0,6%. A Fisher’s exact test is used to calculate for risk factors. Unfortunately this research did not found any significant risk factors for calves to get cryptosporidiosis.
1. Introduction
Cryptosporidium is a protozoan parasite, which has a monogenous lifecycle. It is capable of infecting different vertebrate hosts, including humans, livestock and wild animals all over the world (Castro-Hermida et al. 2002, 11-17). The Cryptosporidium genus contains at least 20 species, but only four of these species have been described in cattle; C. parvum , C. bovis , C. andersoni and C. ryanae . Cryptosporidium parvum can infect humans and is known for its zoonotic potential(Chalmers 2014, 287-326; Rieux et al. 2013, 7-12). Infection with
Cryptosporidium spp . usually causes a self-limiting diarrhea in cattle, but it can be fatal for calves and other immunocompromised animals. Young calves are showing the highest prevalence for Cryptosporidium spp . and they also have the highest intension of shedding oocysts. Among all the different Cryptosporidium spp . described in cattle, pre-weaned calves excrete mainly C. parvum (Brook et al. 2008a, 46-52) . Among humans, young children and immunocompromised people, such as HIV positive patients, may develop severe and even fatal Cryptosporidium infections (Xiao and Feng 2008, 309-323) .
Considering that close contact with cattle has previously been identified as a significant risk factor for humans to become infected with Cryptosporidium oocysts (Wegayehu, Adamu, and
Petros 2013, 419) and that the prevalence of HIV/AIDS in rural South Africa is amongst the highest in the world (34%) (Moshabela et al. 2011, 842-852) it is to be expected that cryptosporidiosis will be especially a risk for humans living in rural farming areas, such as the
Mnisi Traditional Authority. However there are already studies looking for the prevalence and the risk factors of Cryptosporidium in Africa. In South Africa there are only several studies for the prevalence of Cryptosporidium, but there are no studies focusing on risk factors for calves to get cryptosporidiosis in South-Africa.
In Africa there have been several studies performed to find the prevalence of Cryptosporidium in cattle. These studies show that there are differences between these prevalence’s. A study in
Egypt showed that the prevalence of C. parvum in pre weaned calves was 43,5% (Amer et al.
2013, 518-523) . Other studies showed that in certain areas in Kenya and Nigeria the prevalence of Cryptosporidium in cattle is respectively 7,7% and 33% (Kang'ethe et al. 2012,
25-31; Faleke et al. 2014, 443-446). Another study performed in Zambia shows that the overall prevalence of Cryptosporidium in calves from different types of farms is 19,2%
(Geurden et al. 2006b, 217-222).
In Africa, there have also been a number of studies to identify risk factors for calves to get cryptosporidiosis. In Zambia researchers have found that the way of cattle husbandry can be a risk factor (Geurden et al. 2006b, 217-222) . The prevalence in traditional cattle husbandry
(6,3%) was significant lower than the prevalence in dairy husbandry (42,8%). In Egypt one study identified that the “source of drinking water” can be a risk factor for cattle and buffalo
(Helmy et al. 2013, 15-24) . Animals watered with tap water (19,8%) had a significant lower prevalence of Cryptosporidium than animals watered with underground or canal water
(80,2%).
In South Africa there have been a few studies for Cryptosporidium in cattle. Reseachers (Abu
Samra et al. 2013, 295-302) found that the prevalence of Cryptosporidium in calves from traditional husbandry, ranging from 6 months to 1 year of age, was 7,8%. So far, there are no studies for identifying risk factors in cattle performed in South Africa.
Several other studies in the world have shown that young calves are more often affected with cryptosporidiosis, than older cows. In Denmark, it has been shown that there is an age-related herd prevalence of 96% for calves younger than one month. The other age related herd prevalences of adult cows and calves from 1 – 12 months were significantly lower (Maddox-
Hyttel et al. 2006, 48-59). On the other hand in Norway it was found that the infection peak of a cryptosporidium infection was at the age of 2 to 3 months (Hamnes, Gjerde, and Robertson
2006, 204-216). Other studies have shown that the highest prevalence of infection was at two weeks of age and also that the prevalence of Cryptosporidium in pre-weaned calves is higher
(Santín, Trout, and Fayer 2008, 15-23; Fayer et al. 2006, 105-112).
Our research is focused on cryptosporidiosis in a rural area in Mpumalanga, South Africa.
The aim of the research was to estimate the prevalence of Cryptosporidium in young calves and to identify risk factors for calves to get infected with Cryptosporidium . Box 1. Total amount of cattle owners and cattle for each diptank
Diptank Cattle owners Total Cattle
Athol 110 1009 2. Materials and methods
2.1 Area description
This study was performed in an area next to
Kruger National Park called the Mnisi
Traditional Authority. This region comprised eleven diptanks. In this area, livestock
Clare A
Clare B
Gottenburg
Islington
Ludlow
57
91
108
-
-
738
1087
1046
-
- owners are mandatory to bring their cattle once a week to a diptank. Here the cattle have to be dipped to protect them against
Share
Shorty
63
52
743
536
Thlavekisa 64 606 tick-borne diseases. In addition, the cows are randomly checked for foot and mouth disease. Box 1 presents the estimated amount of livestock owners and cattle at the time of
Welverdiend A
Welverdiend B
Total
152
81
778
1807
853
8425
this research in 2012. Unfortunately due to disorganized paper work in Ludlow and Islington, there were no numbers available of these diptanks. Because of a lack in ear tags and good administration in this reagion, individual calves could not be identified. To reduce the chance of sampling the same calf twice, we visited every diptank only one time. The most common cattle breed in Mnisi area is the Nguni breed (Simela 2012) .
2.2 Selection of participants
Every calf less than six months old is a potential candidate for this research. Participation in this study was completely voluntary for the livestock owners. At the diptank the farmers were asked for their participation in this study. This permission was given orally, because most people in this region are illiterate. For these conversations a translator was hired to translate to and from the Shangaan language. After the permission of the livestock owners we were able to take samples.
2.3 Questionnaire
A short questionnaire consisting of five questions was subjected to each participating farmer.
This survey was conducted by the translator. The questions were about to get insight in the living conditions of the participating calves. Closed questions and open questions were included. The two open questions were about which diptank and the herd size the calf was in.
For the age of the calf, man had to choose between an age of <1 month, 1-2 months, 2-4 months, 4-6 months and >6 months and for the water source man had to choose between the dam, tap water and river water. The researchers themselves completed the remaining two questions about the body condition score (BCS) and the consistency of the feces from the sampled calves. The BCS of the calves was determined on a derivative 1 to 5 scale (1= emaciated and 5= obese) with 1.0 increment (Ferguson, Galligan, and Thomsen 1994, 2695-
2703). The fecal consistency was classified in watery, soft and solid. This questionnaire is designed to try to find out about a possible connection between cryptosporidiosis and one of these parameters.
2.4 Sampling
In the morning, freshly fecal samples were collected rectally from pre-weaned calves using a disposable latex glove. The samples were placed in sterile containers. These containers were each numbered with a unique code and stored in a cooler box. In the afternoon the morning samples were diluted with 2,5% potassium dichromate and stored at 4 C. At the end of the collecting period, all the samples together were sent in a portable fridge to the National
Institute for Communicable Diseases (NCID) in Johannesburg for sample analysis.
2.5 Sample analysis
All the fecal samples were concentrated by the formalin- ethyl acetate sedimentation method.
The concentrated samples were stained using the modified Ziehl-Neelsen method (MZN).
These stained samples were examined by light microscopy using the 50x and 100x objectives to detect Cryptosporidium spp.
oocysts.
3. Results
A sample of 354 calves is randomly selected in this population area. In this research, there are
2 calves with a positive MZN staining for Cryptosporidium , see figure x. The estimated population proportion of calves infected with Cryptosporidium is thus p = 2/354 = 0,0056
(0,56%).
The 95% confidence interval for π is calculated by p ± 1,96√( p (1p )/ n ). In this study the 95% confidence interval is (0.000;0.013). Assuming that the sensitivity of the method used for detecting Cryposporidium in this study is 100%. One can say certainly with 95% confidence that the true prevalence for Cryptosporidium in calves in this study area is between the 0 and
1,3%.
Several binomial distributions for different prevalence’s are shown in Table 1.
Table 1 x 0,0056 0,01 0,02 0,03
0
1
2
0,137
0,273
0,271
0,029
0,102
0,182
0,001
0,006
0,020
0,000
0,000
0,001
3
4
0,179
0,089
0,215
0,191
0,049
0,087
0,005
0,012
5 0,035 0,135 0,125 0,026
As you can see in table 1, there is statistically an acceptable change to pick 2 positive samples out of 354 calves when the prevalence is 0,0056, 0,01, 0,02 (or 0,03).
Graph 1
0,3
0,0056
0,25
0,01
0,2
0,02
0,03
0,15
0,1
0,05
0
0 1 2 3 5 6 7 8
As you can see in graph 1, there is also a chance to pick two positive calves when the prevalence is higher than 0,56%. Assuming that the sensitivity of this method is 100% it still could be that the found prevalence is higher. As shown in table 1 there is acceptable chance
(0,18) to pick two positive calves when the prevalence is 1% and even a small change (0,02) when the prevalence is 2%. More on this will be discussed in the discussion.
Table 2
Calf no.
WA 4
Diptank Age of the calf
Welverdiend A 2-3 months
I 16 Islington
Fecal consistency
Solid
1 month Soft
BCS
2-3
2-3
Source of the water
Dam
Dam
Herdsize
17
17
As shown in table 2, the two calves that tested positive have similarities and differences in their questionnaires. Unfortunately due to the low amount of positive samples that are found, it is almost not possible to calculate for risk factors in this case. Given the fact that age is the most frequently studied risk factor for Cryptosporidium spp.
in literature(Maddox-Hyttel et al.
2006, 48-59; Hamnes, Gjerde, and Robertson 2006, 204-216). Therefore we will see if there is in this case a significant relationship between the age of the calves and having cryptosporidiosis. Therefore our hypothesis is:
H
0
: There is no relationship between the age of calves and having cryptosporidiosis.
H
1
: There is a relationship between the age of calves and having cryptosporidiosis
Fisher’s exact test:
Case Processing Summary
Cases
Age * Disease
N
Valid
Percent
347 100,0%
N
Missing
Percent
0 0,0%
N
Total
Percent
347 100,0%
Age
Total
< 1 month
1-2 months old
2-4 months old
4-6 months old
Age * Disease Crosstabulation
Disease
Count
% within Age
% within Disease
Count
% within Age
% within Disease
Count
% within Age
% within Disease
Count negative positive
34 0
100,0%
9,9%
51
98,1%
14,8%
185
99,5%
53,6%
75
100,0%
0,0%
0,0%
1
1,9%
50,0%
1
0,5%
50,0%
0
0,0% % within Age
% within Disease
Count
21,7%
345
0,0%
2
% within Age
% within Disease
99,4%
100,0%
0,6%
100,0%
100,0%
53,6%
75
100,0%
21,6%
347
100,0%
100,0%
Total
34
100,0%
9,8%
52
100,0%
15,0%
186
Chi-Square Tests
Pearson Chi-Square
Likelihood Ratio
Fisher's Exact Test
Linear-by-Linear Association
N of Valid Cases
Value
2,282 a
2,284
2,452
,371 b
347 df
3
3
1
Asymp. Sig. (2sided)
,516
,516
,542
Exact Sig. (2sided)
,481
,768
,481
Exact Sig. (1sided)
,648 ,370
Point Probability
,204 a. 4 cells (50,0%) have expected count less than 5. The minimum expected count is ,20. b. The standardized statistic is -,609.
As you can see above, the p-value for the fisher’s exact test is 0,481. Because p > 0,05 we cannot reject our null hypothesis and we can conclude that there is no significant relation found between age and having cryptosporidiosis in this study. Unfortunately due to the low positive samples, all the parameters were not significant risk factors. The amount of calves used for the Fisher’s exact test is 347. This is because of 7 calves from which the age was unknown.
4. Discussion
Compared to the other studies conducted in Africa, this study showed a relatively low prevalence of 0.6%, especially when compared to a study, which has been performed in an area adjacent to our study area (Abu Samra et al. 2013, 295-302). They found that four of the
51 stool samples (8%; 95% C.I. 2.2, 18.9%) were positive for Cryptosporidium . The discrepancy between the prevalence of this study and the other prevalence’s may be due to various reasons.
The sampling method used in the current study is the same as they use in other studies. Other studies also collected the samples rectally by using latex gloves and sterile sample containers
(Abu Samra et al. 2013, 295-302; Hamnes, Gjerde, and Robertson 2006, 204-216; Brook et al.
2008a, 46-52) . The way of which the samples were taken is probably not the point of difference.
One possible explanation may be due to the time of our sampling activities; sampling started in mid-March, just after calving season, which is known to last from December to the beginning of March in South Africa. It has been reported in previous studies that oocysts of
Cryptosporidium spp.
are mainly shed by calves of very young age (Nydam et al. 2001, 1612-
5; Santín, Trout, and Fayer 2008, 15-23) and that the number of oocysts shed increased with the age of the calves until approximately 12 days, after which it decreases. In fact, the age of the calves sampled in the present study was on average between 2 to4 months. This fact may have possibly led to low amounts of oocysts excreated in the fecal samples and therefore, making it difficult to detect low number of Cryptosporidium oocysts by microscopy.
For the diagnosis of Cryptosporidium in this research the Modified Ziehl Neelsen (MZN) staining method was used to detect oocysts in de fecal samples. The MZN technique is a good detecting method to look for cryptosporidiosis, which is widely available and low in costs, but they need microscopic skills and a proper staining (Checkley et al. 2015, 85-94; Ramirez,
Ward, and Sreevatsan 2004, 773-785) . However, when the amount of oocysts in de the fecal sample is low, it is harder to detect the oocysts (Brook et al. 2008b, 26-31) .
In the literature there are different values given for the sensitivity and specificity for the MZN staining method. For example (Brook et al. 2008b, 26-31) report that the sensitivity of the
MZN staining method in fresh fecal samples is 83% and the sensitivity is 88%. Another study in Saudi Arabia reports a sensitivity and specificity of respectively 73,3% and 95% (Zaglool et al. 2013, 212-215) and in yet another study researchers claim that the sensitivity of MZN staining method is between the 70% and 80% (Checkley et al. 2015, 85-94) .
Next to the MZN staining method there are other diagnostic tools to detect Cryptosporidium in fecal samples. For example PCR, which has a higher sensitivity than microscopically examination (Geurden et al. 2006a, 671 - 682) . In the United Kingdom, researchers reported a sensitivity of 100% and a specificity of 99,1% (Hadfield et al. 2011, 918-924) . Other studies, which are similar to the present study, have used PCR for detecting Cryptosporidium
(Abu Samra et al. 2013, 295-302; Kang'ethe et al. 2012, 25-31) and they identify more positive fecal samples with Cryptosporidium after PCR than they had after microscopic examintation.
Returning to Table 1 and Graph 1. These show the chance to pick 2 positive calves at different prevalence’s. These binomial distributions are calculated with n= 354, p=(0,0056;
0,01; 0,02; 0,03; 0,04) and the variable is the amount of positive calves. In this study we found 2 positive samples. Because we did not sample the whole population, there is still a change that a majority of positive samples were not sampled. In the case that our sensitivity is not 100% you also have a chance that we have missed positive samples. The true population of positive calves is than higher than 0,0056. In the end we don’t know what the sensitivity and specificity of the microscopic examination in this study is, because the human interpretation plays a major role (Checklet et al. 2015, 85-94; Ramirez, Ward, and Sreevatsan
2004, 773-785) .
Let’s assume that the sensitivity and specificity of our microscopic examination was respectively 40% and 80% (Checkley et al. 2015, 85-94; Geurden et al. 2006a, 671 - 682) .
Than you can say that 40% is false negative and 20% is false positive. Which means that
(2/3) 2 samples were true positive and (1/3) 2 samples were false positive.
If this study will be repeated, you should calculate the sample size you need for significant results. For example you can use the following formula: n= Z 2 P(1-P)
d 2
With Z = Z statistic for a level of confidence, P = expected prevalence or proportion, and d = precision (Naing, Winn, and Rusli 2006, http://www.kck.usm.my/ppsg/aos/Vol_1/09_14_Ayub.pdf
) . In this case for a 95% confidence interval Z = 1,96, P = 0,0056 and d = ( P /2) = 0,0028. When you fill the formula in, than the result is n = 2729. Meaning that you might need 2729 calves or cattle to do some proper statistical analysis the next time.
Briefly we can say that in this study a low prevalence and no significant risk factors are found. And it is possible that this is due to the differences as described above. However, it might be that the prevalence of Cryptosporidium spp . at that time was not that high anyway.
Next time one should try to do PCR on all the samples. If then there are more positive calves, then it is possible to do some proper statistic work to look for significant risk factors.
6. Literature
Abu Samra, Nada, Ferran Jori, Lihua Xiao, Oupa Rikhotso, and Peter N. Thompson. 2013. "Molecular
Characterization of Cryptosporidium Species at the Wildlife/Livestock Interface of the Kruger National
Park, South Africa." Comparative Immunology, Microbiology and Infectious Diseases 36 (3): 295-302. doi: http://dx.doi.org.proxy.library.uu.nl/10.1016/j.cimid.2012.07.004
.
Amer, Said, Shereif Zidan, Haileeyesus Adamu, Jianbin Ye, Dawn Roellig, Lihua Xiao, and Yaoyu Feng. 2013.
"Prevalence and Characterization of Cryptosporidium Spp. in Dairy Cattle in Nile River Delta Provinces,
Egypt." Experimental Parasitology 135 (3): 518-523. doi: http://dx.doi.org/10.1016/j.exppara.2013.09.002
.
Brook, E., C. A. Hart, N. French, and R. Christley. 2008a. "Prevalence and Risk Factors for Cryptosporidium
Spp. Infection in Young Calves." Veterinary Parasitology 152 (1-2): 46-52.
Brook, E. J., R. M. Christley, N. P. French, and C. A. Hart. 2008b. "Detection of Cryptosporidium Oocysts in
Fresh and Frozen Cattle Faeces: Comparison of Three Methods." Letters in Applied Microbiology 46 (1):
26-31. doi:10.1111/j.1472-765X.2007.02257.x.
Castro-Hermida, José A., Yolanda A. González-Losada, Mercedes Mezo-Menéndez, and Elvira Ares-Mazás.
2002. "A Study of Cryptosporidiosis in a Cohort of Neonatal Calves." Veterinary Parasitology 106 (1): 11-
17. doi:10.1016/S0304-4017(02)00038-9.
Chalmers, Rachel M. 2014. "Chapter Sixteen - Cryptosporidium." In Microbiology of Waterborne Diseases
(Second Edition) , edited by Steven L. Percival, Marylynn V. Yates, David W. Williams, Rachel M.
Chalmers and Nicholas F. Gray, 287-326. London: Academic Press. doi: http://dx.doi.org/10.1016/B978-0-
12-415846-7.00016-0 . http://www.sciencedirect.com/science/article/pii/B9780124158467000160 .
Checkley, William, A. Clinton White Jr, Devan Jaganath, Michael J. Arrowood, Rachel M. Chalmers, Xian-
Ming Chen, Ronald Fayer, et al. 2015. "A Review of the Global Burden, Novel Diagnostics, Therapeutics, and Vaccine Targets for Cryptosporidium." The Lancet Infectious Diseases 15 (1): 85-94. doi: http://dx.doi.org/10.1016/S1473-3099(14)70772-8 .
Faleke, O. O., Y. A. Yabo, A. O. Olaleye, Y. U. Dabai, and E. B. Ibitoye. 2014. "Point Prevalence of
Cryptosporidium Oocyst in Calves Grazing Along River Rima Bank in Sokoto, Nigeria." Pakistan Journal of Biological Sciences 17 (3): 443-446.
Fayer, R., M. Santín, J. M. Trout, and E. Greiner. 2006. "Prevalence of Species and Genotypes of
Cryptosporidium found in 1-2-Year-Old Dairy Cattle in the Eastern United States." Veterinary
Parasitology 135 (2): 105-112.
Ferguson, James D., David T. Galligan, and Neal Thomsen. 1994. "Principal Descriptors of Body Condition
Score in Holstein Cows." Journal of Dairy Science 77 (9): 2695-2703. doi: http://dx.doi.org.proxy.library.uu.nl/10.3168/jds.S0022-0302(94)77212-X .
Geurden, T., D. Berkvens, P. Geldhof, J. Vercruysse, and E. Claerebout. 2006a. "A Bayesian Approach for the
Evaluation of Six Diagnostic Assays and the Estimation of Cryptosporidium prevalence in Dairy Calves."
Vet. Res.
37 (5): 671 - 682.
Geurden, T., F. Y. Goma, J. Siwila, I. G. K. Phiri, A. M. Mwanza, S. Gabriel, E. Claerebout, and J. Vercruysse.
2006b. "Prevalence and Genotyping of Cryptosporidium in Three Cattle Husbandry Systems in Zambia."
Veterinary Parasitology 138 (3–4): 217-222. doi: http://dx.doi.org/10.1016/j.vetpar.2006.02.009
.
Hadfield, Stephen J., Guy Robinson, Kristin Elwin, and Rachel M. Chalmers. 2011. "Detection and
Differentiation of Cryptosporidium Spp. in Human Clinical Samples by use of Real-Time PCR." Journal of
Clinical Microbiology 49 (3): 918-924. doi:10.1128/JCM.01733-10.
Hamnes, Inger Sofie, Bjørn Gjerde, and Lucy Robertson. 2006. "Prevalence of Giardia and Cryptosporidium in
Dairy Calves in Three Areas of Norway." Veterinary Parasitology 140 (3–4): 204-216. doi: http://dx.doi.org.proxy.library.uu.nl/10.1016/j.vetpar.2006.03.024
.
Helmy, Yosra A., Jürgen Krücken, Karsten Nöckler, Georg von Samson-Himmelstjerna, and Karl-H Zessin.
2013. "Molecular Epidemiology of Cryptosporidium in Livestock Animals and Humans in the Ismailia
Province of Egypt." Veterinary Parasitology 193 (1–3): 15-24. doi: http://dx.doi.org/10.1016/j.vetpar.2012.12.015
.
Kang'ethe, E. K., E. K. Mulinge, R. A. Skilton, M. Njahira, J. G. Monda, C. Nyongesa, C. K. Mbae, and S. K.
Kamwati. 2012. "Cryptosporidium Species Detected in Calves and Cattle in Dagoretti, Nairobi, Kenya."
Tropical Animal Health and Production 44 (SUPPL.1): 25-31.
Maddox-Hyttel, Charlotte, Rikke B. Langkjær, Heidi L. Enemark, and Håkan Vigre. 2006. "Cryptosporidium and Giardia in Different Age Groups of Danish Cattle and pigs—Occurrence and Management Associated
Risk Factors." Veterinary Parasitology 141 (1–2): 48-59. doi: http://dx.doi.org.proxy.library.uu.nl/10.1016/j.vetpar.2006.04.032
.
Moshabela, M., P. Pronyk, N. Williams, H. Schneider, and M. Lurie. 2011. "Patterns and Implications of
Mecical Pluralism among HIV/AIDS Patients in Rural South Africa." AIDS and Behavior : 842-852.
Naing, L., T. Winn, and B. N. Rusli. 2006. "<br />Practical Issues in Calculating the Sample Size for Prevalence
Studies ." : http://www.kck.usm.my/ppsg/aos/Vol_1/09_14_Ayub.pdf
.
Nydam, D. V., S. E. Wade, S. L. Schaaf, and H. O. Mohammed. 2001. "Number of Cryptosporidium Parvum
Oocysts Or Giardia Spp Cysts Shed by Dairy Calves After Natural Infection." American Journal of
Veterinary Research 62 (10): 1612-5.
Ramirez, Norma E., Lucy A. Ward, and Srinand Sreevatsan. 2004. "A Review of the Biology and Epidemiology of Cryptosporidiosis in Humans and Animals." Microbes and Infection 6 (8): 773-785. doi: http://dx.doi.org.proxy.library.uu.nl/10.1016/j.micinf.2004.02.021
.
Rieux, Anaïs, Carine Paraud, Isabelle Pors, and Christophe Chartier. 2013. "Molecular Characterization of
Cryptosporidium Isolates from Pre-Weaned Calves in Western France in Relation to Age." Veterinary
Parasitology 197 (1–2): 7-12. doi: http://dx.doi.org/10.1016/j.vetpar.2013.05.001
.
Santín, Mónica, James M. Trout, and Ronald Fayer. 2008. "A Longitudinal Study of Cryptosporidiosis in Dairy
Cattle from Birth to 2 Years of Age." Veterinary Parasitology 155 (1–2): 15-23. doi: http://dx.doi.org.proxy.library.uu.nl/10.1016/j.vetpar.2008.04.018
.
Simela, L. et al. 2012. "Options for the Delivery of Primary Animal Health Care for Livestock Farmers on
Communal Land in South Africa: Mnisi Community Case Study ."University of Pretoria.
Wegayehu, Teklu, Haileeyesus Adamu, and Beyene Petros. 2013. "Prevalence of Giardia Duodenalis and
Cryptosporidium Species Infections among Children and Cattle in North Shewa Zone, Ethiopia." BMC
Infectious Diseases 13: 419. doi:10.1186/1471-2334-13-419.
Xiao, Lihua and Yaoyu Feng. 2008. "Zoonotic Cryptosporidiosis." FEMS Immunology & Medical Microbiology
52 (3): 309-323. doi:10.1111/j.1574-695X.2008.00377.x.
Zaglool, Dina Abdulla Muhammad, Amr Mohamed, Yousif Abdul Wahid Khodari, and Mian Usman Farooq.
2013. "Crypto-Giardia Antigen Rapid Test Versus Conventional Modified Ziehl-Neelsen Acid Fast
Staining Method for Diagnosis of Cryptosporidiosis." Asian Pacific Journal of Tropical Medicine 6 (3):
212-215. doi: http://dx.doi.org/10.1016/S1995-7645(13)60025-5 .