Unit 7 Homework - NGHS
advertisement

Name:
AP Chemistry: Unit 7: Chemical Bonding: Homework
Date:
1. In general terms, how does each of the following atomic properties influence the metallic character
of the main-group elements in a period?
a. Ionization energy
b. Atomic radius
c. Number of outer electrons
d. Effective nuclear charge
2. State whether the type of bonding in the following compounds is best described as ionic or
covalent: (a) CsF(s); (b) N 2(g); (c) H2S(g).
3. Use condensed electron configurations and Lewis electron- dot symbols to depict the monatomic
ions formed from each of the following atoms, and predict the formula of the compound the ions
produce: (a) Ba and Cl (b) Sr and O (c) Al and F (d) Rb and O
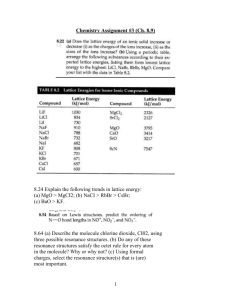
4. For each pair, choose the compound with the lower lattice energy, and explain your choice:
(a) CaS or BaS (b) NaF or MgO
5. Define bond energy using the H—Cl bond as an example. When this bond breaks, is energy absorbed
or released, is the accompanying ΔH value positive or negative? How do the magnitude and sign of
this ΔH value relate to the value that accompanies H —Cl bond formation?
6. Using the periodic table only, arrange the members of each of the following sets in order of increasing
bond strength:
(a) Br-Br, Cl-Cl, I-I
(b) S-H, S-Br, S-C1
(c) C=N, C-N, C≡N
7. Which gas has the greater heat of reaction per mole of combustion? Why?
methane
formaldehyde
H
O
|
||
H—C—H
H—C—H
|
H
8. Is the H-O bond in water nonpolar covalent, polar covalent, or ionic? Define each term, and explain
your choice.
9. An important industrial route to extremely pure acetic acid is the reaction of methanol with
carbon monoxide as shown below. Use bond energies to calculate the heat of reaction.
H
H O
|
| ||
H—C—O—H + C≡O H—C—C—O—H
|
| |
H
H H
10. Using the periodic table only, arrange the elements in each set in order of increasing EN: (a) S, O, Si;
(b) Mg, P, As.
11. Are the bonds in each of the following substances ionic, non- polar covalent, or polar covalent?
Arrange the substances with polar covalent bonds in order of increasing bond polarity: (a) S8
(b) RbCl
(c) PF3
(d) SC12
(e) F2
(f) SF2
12. Rank the members of each set of compounds in order of increasing ionic character of their bonds.
Use a polar arrow to indicate the bond polarity of each: (a) HBr, HCl, HI
(b) H2O, CH4, HF
(c) SCl2, PCl3, SiCl4
13. Even though so much energy is required to form a metal cation with a 2+ charge, the alkaline
earth metals form halides with general formula MX2, rather than MX.
(a) Use the following data to calculate the ΔHf° of MgCl:
Mg(s) Mg(g)
ΔH° = 148 kJ
C12(g) 2C1(g)
ΔH° = 243 kJ
Mg(g) Mg(g) + e
ΔH° = 738 kJ
Cl(g) + e Cl (g)
ΔH° = -349 kJ
ΔH lattice of MgCl = 783.5 kJ/mol
(b) Is MgCl favored energetically relative to its elements? Explain.
(c) Use Hess's law to calculate ΔH° for the conversion of MgCl to MgCl2 and Mg (ΔH f °of MgC12 = 641.6 kJ/mol).
(d) Is MgC1 favored energetically relative to MgC12? Explain.
14. By using photons of specific wavelengths, chemists can dissociate gaseous HI to produce H atoms
with certain speeds. When HI dissociates, the H atoms move away rapidly, whereas the relatively
heavy I atoms move more slowly.
(a) What is the longest wavelength (in nm) that can dissociate a molecule of HI?
(b) If a photon of 254 nm is used, what is the excess energy (in I) over that needed for the
dissociation?
(c) If all this excess energy is carried away by the H atom as kinetic energy, what is its speed (in
m/s)?
15. Without stratospheric ozone (O3), harmful solar radiation would cause gene alterations. Ozone forms
when O2 breaks an, each O atom reacts with another O2 molecule. It is destroyed by reaction with Cl
atoms that are formed when the C—Cl bond in synthetic chemicals breaks. Find the wavelengths of
light that can break the C —Cl bond and the bond in O2.
16. "Inert" xenon actually forms many compounds, especially with highly electronegative fluorine. The
AHf° values for xenon difluoride, tetrafluoride, and hexafluoride are -105, -284, and -402 kJ/mol,
respectively. Find the average bond energy of the Xe—F bonds in each fluoride.
17. There are two main types of covalent bond breakage. In homolytic breakage, each atom in the bond
gets one of the shared electrons. In some cases, the electronegativity) of adjacent atoms affects the
bond energy. In heterolytic breakage, one atom gets both electrons and the other gets none; thus, a
cation and an anion form.
a. Why is the C—C bond in H3C—CF3 (423 kJ/mol) stronger than the bond in H3C —CH3 (376
kJ/mol)?
b. Use bond energy and any other data to calculate the heat of reaction for the heterolytic
cleavage of O2.
18. Which of these atoms cannot serve as a central atom in a Lewis structure: (a) O; (b) He; (c) F;
(d) H; (e) P? Explain.
19. Draw a Lewis structure for (a) SiF4; (b) SeC12; (c) COF2 (C is central).
20. Draw a Lewis structure for (a) PF3; (b) H2CO3 (both H atoms are attached to O atoms); (c) CS2.
21. Draw Lewis structures of all the important resonance forms of (a) NO2+; (b) NO2F (N is central).
22. Draw a Lewis structure and calculate the formal charge of each atom in (a) IF5; (b) AlH4–.
23. These species do not obey the octet rule. Draw a Lewis structure for each, and state the type of
octet-rule exception:
(a) BH3
(b) AsF4–
(c) SeCl4
24. Molten beryllium chloride reacts with chloride ion from molten NaCl to form BeCl42- ion, in which
the Be atom obtains an octet. Show the net ionic reaction with Lewis structures.
25. Determine the electron-group arrangement, molecular shape, and ideal bond angle(s) for each of the
following:
(a) O3 (b) H3O+ (c) NF3
26. Determine the electron-group arrangement, molecular shape, and ideal bond angle(s) for each of the
following:
('a) CO32–
(b) SO2
(c) CF4
27. Determine the shape, ideal bond angle(s), and the direction k)f any deviation from these angles for
each of the following:
(a) C1O2– (b) PF5
(c) SeF4
(d) KrF2
28. Arrange the following AFn species in order of increasing F—A—F bond angles: BF3, BeF2, CF4, NF3,
OF2.
29. In the gas phase, phosphorus pentachloride exists as separate molecules. In the solid phase, however,
the compound is composed of alternating PCl4+ and PCl6– ions. What change(s) in molecular shape
occur(s) as PC15 solidifies? How does the CI—P—C1 angle change?
30. Consider the molecules SC12, F2, CS2, CF4, and BrCl.
(a) Which has bonds that are the most polar?
(b) Which have a molecular dipole moment?
31. Which molecule in each pair has the greater dipole moment? Give the reason for your choice.
(a) SO2 or SO3 (b) ICl or IF
(a) SiF 4 or SF 4 (d) H2O or H2S
32. In addition to ammonia, nitrogen forms three other hydrides: hydrazine (N2H4), diazene (N2H2), and
tetrazene (N4H4).
a. Use Lewis structures to compare the strength, length, and order of nitrogen-nitrogen bonds in
hydrazine, diazene, and N2.
b. Tetrazene (atom sequence H2NNNNH2) decomposes above 0°C to hydrazine and nitrogen gas.
Draw a Lewis structure for tetrazene, and calculate ΔH°for this decomposition.
33. Ethanol (CH3CH2OH) is being used as a gasoline additive or alternative in many parts of the world.
a. Use bond energies to find the ΔH° for the combustion gaseous ethanol. (Assume H2O forms
as a gas.)
b. In its standard state at 25°C, ethanol is a liquid. Its vaporization requires 40.5 kJ/mol. Correct
the value from part (a) to find the heat of reaction for the combustion of liquid ethanol.
c. How does the value from part (b) compare with the value you calculate from standard heats of
formation (Appendix B in book)?
d. Methods for sustainable energy produce ethanol from corn and other plant material, but the
main industrial method still involves hydrating ethylene from petroleum. Use Lewis structures
and bond energies to calculate ΔH° for the formation of gaseous ethanol from ethylene gas with
water vapor.
34. A molecule of formula AY 3 is found experimentally to be polar. Which molecular shapes are
possible and which impossible for AY3?
35. Give the number and type of hybrid orbital that forms when each set of atomic orbitals mixes:
(a) two d, one s, and three p
(b) three p and one s
36. What is the hybridization of nitrogen in each of the following: (a) NO; (b) NO2; (c) NO2-?
37. What is the hybridization of chlorine in each of the following: (a) C1O2; (b) C1O3-; (c) C1O4-?
38. Which types of atomic orbitals of the central atom mix to form hybrid orbitals in (a) SiC1H3; (b) CS2?
39. Phosphine (PH3) reacts with borane (BH3) as follows:
PH3 + BH3 H3P -BH3
a. Which of the illustrations below depicts the change, if any, in the orbital hybridization of P during
this reaction?
b. Which depicts the change, if any, in the orbital hybridization of B?
40. Use partial orbital diagrams to show how the atomic orbitals of the central atom lead to hybrid orbitals
in (a) GeC14; (b) BC13; (c) CH3C1.
41. Use partial orbital diagrams to show how the atomic orbitals of the central atom lead to hybrid
orbitals in (a) SeC1 2; (b) H3O+; (c) IF5.
42. Are these statements true or false? Correct any that are false.
a. Two σ bonds comprise a double bond.
b. A triple bond consists of one π bond and two σ bonds.
c. Bonds formed from atomic s orbitals are always σ bonds.
d. A π bond restricts rotation about the σ-bond axis.
e. A π bond consists of two pairs of electrons.
f. End-to-end overlap results in a bond with electron density above and below the bond axis.
43. Describe the hybrid orbitals used by the central atom and the type(s) of bonds formed in (a) NO3-; (b)
CS2; (c) CH2O.
44. Describe the hybrid orbitals used by the central atom(s) and the type(s) of bonds formed in (a) FNO;
(b) C2F4; (c) (CN)2.
45. How do the bonding and antibonding MOs formed from a given pair of AOs compare to each other
with respect to (a) energy; (b) presence of nodes; (c) internuclear electron density?
46. How many electrons does it take to fill (a) a σ bonding MO; (b) a π antibonding MO; (c) the MOs
formed from combination of the 1s orbitals of two atoms?
47. Use MO diagrams and the bond orders you obtain from them to answer: (a) Is Be2+ stable? (b) Is
Be2+ diamagnetic? (c) What is the outer (valence) electron configuration of Be2+?
48. Epinephrine (or adrenaline) is a naturally occurring hormone that is also manufactured commercially
for use as a heart stimulant, a nasal decongestant, and to treat glaucoma. A valid Lewis structure is
a. What is the hybridization of each C, O, and N atom')
b. How many σ bonds does the molecule have?
c. How many π electrons are delocalized in the ring?
49. Acetylsalicylic acid (aspirin), the most widely used medicine in the world, has the following Lewis
structure:
a. What is the hybridization of each C and each O atom?
b. How many localized π bonds are present?
c. How many C atoms have a trigonal planar shape around them? A tetrahedral shape?
50. Fill in the blank information.
Molecule/Ion
Lewis Structure
Electron Pair Geometry: Hybridization:
Molecular Geometry:
Bond Order:
Polar or Nonpolar:
Resonance Forms
(Y/N)?
SeI2
Molecule/Ion
Lewis Structure
Electron Pair Geometry: Hybridization:
Molecular Geometry:
Bond Order:
Polar or Nonpolar:
Resonance Forms
(Y/N)?
CO32-
51. Complete the following sections using Molecular Orbital Theory. Draw a complete Molecular
Orbital diagram to answer these questions (include all 1s and 2s interactions, no short hand
notation) and provide the missing information.
Molecule/Ion: Li2
Molecular Orbital Diagram:
Bond Order: ______
Number of sigma bonds:______
(Circle) Paramagnetic or Diamagnetic
Number of pi bonds: ______
Should this molecule exist? (Circle) Yes or No
Molecule/Ion: N2
Molecular Orbital Diagram:
Bond Order: ______
Number of sigma bonds:______
(Circle) Paramagnetic or Diamagnetic
Number of pi bonds: ______
Should this molecule exist? (Circle) Yes or No
Molecule/Ion: Ne2
Molecular Orbital Diagram:
Bond Order: ______
Number of sigma bonds:______
(Circle) Paramagnetic or Diamagnetic
Number of pi bonds: ______
Should this molecule exist? (Circle) Yes or No
52. Complete the following sections using both Valence Bond (VB) Theory and Molecular Orbital (MO)
Theory. Use shorthand notation for MO Diagrams. Provide all of the missing information.
Molecule/Ion: CN1Valence Bond Lewis Structure:
Bond Order (VB):
Bond Order (MO):
# Sigma Bonds (VB):
Molecular Orbital Diagram Using Shorthand Notation:
# Sigma Bonds (MO):
# Pi Bonds (VB):
# Pi Bonds (MO):
(VB) Paramagnetic? (circle) Yes
No
(MO) Paramagnetic? (circle) Yes
No
Molecule/Ion: NO (Use the MO Diagram for O, F and Ne on this problem)
Valence Bond Lewis Structure:
Bond Order (VB):
Bond Order (MO):
# Sigma Bonds (VB):
Molecular Orbital Diagram Using Shorthand Notation:
# Sigma Bonds (MO):
# Pi Bonds (VB):
# Pi Bonds (MO):
(VB) Paramagnetic? (circle) Yes
No
(MO) Paramagnetic? (circle) Yes
No
Molecule/Ion: OF-1
Valence Bond Lewis Structure:
Bond Order (VB):
Bond Order (MO):
# Sigma Bonds (VB):
Molecular Orbital Diagram Using Shorthand Notation:
# Sigma Bonds (MO):
# Pi Bonds (VB):
# Pi Bonds (MO):
(VB) Paramagnetic? (circle) Yes
No
(MO) Paramagnetic? (circle) Yes
No
49. Formula
CHClO
C2H6
NO2+
C2O4-2
Lewis Structure
Molecular
Shape and bond
angle (VSEPR)
Geometrically Correct
Drawing
53. Indicate the polarity of each bond with a polar arrow. Use a bond electronegativity chart.
a. N – B
b. N – O
c. C – S
d. S – O
e. N – H
f. Cl – O
54. Sports trainers treat sprains and soreness with ethyl bromide. It is manufactured by reacting
ethylene with hydrogen bromide:
H
H
H H
C=C
+ H – Br H – C – C – H
H
H
H H
Use bond energies (in your lecture) to find the enthalpy change (ΔH) for this reaction.
55. Acetylene gas (HC≡CH) burns with oxygen to produce carbon dioxide and heat. The heat of the
reaction for the combustion of acetylene is 1259 kJ/mol.
a. Calculate the C≡C bond energy.
b. When 500.0 g of acetylene burns, how many kilojoules of heat are given off?
c. How many liters of oxygen at 298K at 18 atm are consumed?
d. How many grams of carbon dioxide are produced?
56. Carbon-carbon bonds form the “backbone” of nearly every organic and biological molecule. The
average bond energy of the C-C bond is 347 kJ/mol. Calculate the frequency and wavelength of the
least energetic photon that can break the C-C bond. In what region of the electromagnetic spectrum
is this radiation?
57. In which of the following bonding patterns is the octet rule obeyed?
a. –X--
b. ׃X--
c.
X
d. ≡X׃
g.
X
h.
..
e.
–X=
f. –X=
..
׃X׃
••
58. Determine the electron group arrangement, molecular shape, and ideal bond angles for each of the
following.
a. O3
b. H3O+
c. NF3
59. Consider the molecules SCl2, F2, CS2, CF4, and BrCl.
a. Which has the most polar bond?
b. Which have a molecular dipole moment?