Photoelectrochemical Formation of Arsenic Sulphide on GaAs
advertisement

activities that are desirable for several
photochemical
applications.
GaAs
spontaneously oxidizes in aqueous media over a
wide range of pH. Passivation of GaAs has been
intensively studied. Sandroff et al. investigated
the inhibition of GaAs corrosion by treatment in
Na2S electrolyte [8]. Since then sulphur
passivation has attracted considerable attention
[9 - 15].
The objective of the present study is to
investigate the optimum conditions of forming a
continuous and stable inhibition film and to
identify its chemical composition.
Photoelectrochemical Formation of
Arsenic Sulphide on GaAs Surface in
Acidified Thiourea Electrolytes
Mahmoud M. Khader*, Amina
S. AlJaber, Noora M. Alshamry,
Thuraiya Haider and Fatima H.
Alemadi
Department of Chemistry &
Earth Sciences, College of Arts
& Sciences, Qatar University,
P.O. 2713, Doha, Qatar
The present article reports the formation of
arsenic sulfide films on GaAs by the
potentiodynamic polarization in thiourea (TU)
containing acidic electrolytes under photoillumination. Oxidation of TU competed with
the oxidation of GaAs itself and formed a film of
arsenic-sulfide on GaAs surface. The chemical
composition of the surface was investigated by
x-ray photoelectron spectroscopy (XPS),
demonstrating the formation of As-sulfide as the
XPS peaks of the As 3d at 42.6 and the S 2p at
162.5 eV were observed. The morphology of the
As-sulfide film was characterized by SEM that
showed the formation of smooth and nonporous
film in TU electrolyte acidified by H2SO4 of
concentration ≥ 0.2 M. The corrosion rate of
GaAs was investigated by electrochemical
impedance spectroscopy that showed significant
inhibition of GaAs dissolution due to the
deposition of As-sulfide film.
Key words: GaAs, corrosion, inhibition,
impedance, As-sulfide passivation, XPS.
2. Experimental:
Electrodes were made of silicon-doped n-GaAs
wafers with a doping density of 2x1016 cm-3
(MCP Ltd). The electrochemical measurements
were carried out in a three- electrode
electrochemical cell with GaAs serving as the
working electrode, Ag/AgCl as the reference
electrode and a Pt wire as the counter electrode.
The GaAs electrode was cleaned by etching in a
mixture of 30% H2O2, 6 M H2SO4 and H2O
(1:1:1 volume) for 5 min. The electrode was
illuminated by a 150 W xenon lamp. In all
experiments the electrochemical path for arsenic
sulfide film deposition was composed of fixed
concentration of 0.8 M TU, and concentrations
of H2SO4 varying from 0.2 to 0.5 M. In the
following discussion, these paths are denoted as
the sulfide deposition paths. All reagents were
analytical grade. All potentials were measured
versus Ag/AgCl. Electrochemical impedance
was measured at fixed DC voltages by applying
AC
signals
with
varied
frequencies.
Electrochemical measurements were carried out
using an Autolab potentiostat equipped with
Nova 1.5 software. Values of the flatband
potentials, Efb, were directly measured for nGaAs and sulfide-coated n-GaAs electrodes in
the dark and under illumination. Mott-Schottky
(M-S) plots of C-2 vs. E were constructed in
order to directly measure Efb values.
*Corresponding author (mmkhader@qu.edu.qa)
1. Introduction
The thermodynamically favoured susceptibility
of GaAs to corrosion in aqueous media remains
the major obstacle in practical applications of
this material for solar energy conversion.
Therefore, new types of corrosion inhibitors as
well as the methods to apply and characterize
them are needed in order to develop a
sustainable GaAs-based photoelectrochemical
cell (PECC). Corrosion of GaAs has been
investigated intensively in the past [1–3 ] and in
recent years [4–7], due to its photochemical
XPS spectra were taken on a Kratos Axis-Ultra
DLD spectrometer at pressures less than 6 x 10-9
Torr. The pass energy for survey spectra was 80
eV and the pass energy for the high resolution
spectra was 20 eV. Casa XPS software was used
1
to measure peak areas and determine the
elemental composition. The binding energy
scale was calibrated to the C 1s peak at 285.0 eV
for identification of elements and for
determining binding energies in the high
resolution peak fits.
The amounts of dissolved arsenic and gallium
ions were determined quantitatively by ICP-MS
(Agilent, 7500Ce) which utilized an octopole ion
guide enclosed in a collision/reaction cell.
surface
elemental
analysis
of
35
± 2.9 for As and 9.3 for S. Surface C and O
were also determined and found to be
47.6±4.6 and 8.1 ± 0.7, respectiveley. The
present surface As abundance is strongly
supported by the literature results that have
showed arsenic being the binding sites for
sulfide deposition [16 -18].
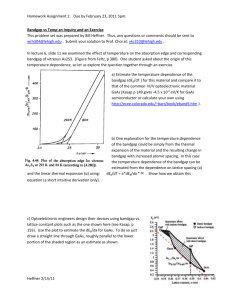
The current - voltage (I – E) behaviour in
contact with the sulfide deposition path is
shown in Fig.2. For comparison, the (I –E)
scans without TU is also plotted in the same
figure. This figure shows that the presence of
TU in the electrolyte enhanced the photocurrents
over the whole range of the potential scans. The
current enhancement is attributed to a decrease
in the surface recombination velocity and an
effective reduction in the nonradioactive
transition processes due to the sulfide
passivation. In an acidified TU electrolyte, the
previous XPS results demonstrated the
formation of As-sulphide on the GaAs surface.
In the meantime, the ICP-MS results, did not
show preferential dissolution of Ga ions into the
electrolyte during the deposition of the Assulphide. Indeed, the ICP-MS always showed a
preferential dissolution of As ions.
3. Results and discussion
Figure 1 shows the XPS spectra of GaAs sample
treated in a mixture of 0.8 M TU and 0.3 M
H2SO4. This figure shows the XPS peaks of As,
S, C and O with no evidence of any Ga XPS
peaks.
30.00
I x 103, A cm-2
25.00
Figure 1: XPS survey spectrum of sample 4
(GaAs was scanned 100 times in 0.3 M H2SO4 +
0.8 M TU).
20.00
15.00
10.00
5.00
0.00
-5.00
-10.00
-1-0.8-0.6-0.4-0.2 0 0.20.40.60.8 1
The high resolution As 3d peak envelope was fit
with a 3d5/2 – 3d3/2 separation of 0.7 eV and an
area ratio of 3/2,. For S, the high resolution of
the 2p peak envelope was fit with a 2p3/2 – 2p1/2
separation of 1.2 eV and an area ratio of 2/1.
From these high resolution XPS analysis,
surface As and S were determined quantitatively
from the fit of the two sets of doublets located at
~41.7 eV and ~42.6 eV for As and the two
doublets at 19.5 eV and 20.6 eV for S [16 -20].
The quantitative analysis showed percentage
E, V (Ag/AgCl)
Figure 2: The fifth current – voltage scans of nGaAs electrode in electrolytes made of 0.1 M
H2SO4 (───) and a mixture of 0.8 M TU + 0.1
M H2SO4 (- - -).
An example of the ICP-MS data after 100 scans
of GaAs in a mixture of 0.8 M TU and 0.3 M
2
H2SO4, the percentage of As and Ga dissolved
were 3.57 ± 0.3 and 2.39 ± 0.33, respectively.
To explain the formation of As-sulfide and, in
the meantime, the preferential dissolution of As
ions, we suggest that GaAs surface was
originally rich in As. It is thus not surprising that
the inhibition film was composed of As-sulphide
only rather than of a mixed As and Ga sulfide.
Fig. 2 shows that in the presence of TU, the
photogenerated currents were about five times
greater than the corresponding photocurrents
generated with H2SO4 alone. This result
furnished a direct evidence for the conversion of
light to electrical energy on n-GaAs electrodes
in TU containing electrolyte; i.e., hole transfer
from the illuminated GaAs electrode surface to
the adsorbed TU molecules was accomplished
using less electrical energy relative to the H2SO4
electrolyte. Another advantage of the Assulfide- coated GaAs as there was no direct
contact between semiconductor and electrolyte
and thus, the semiconductor was protected
against photocorrosion and against other
electrochemical changes which might lead to
recombination
losses.
When
H2SO4
concentration was increased to 0.2 and 0.3 M, a
golden, smooth and nonporous As-sulfide was
obtained, as shown in the SEM micrograph of
Fig. 3. In TU, if the concentration of H2SO4 was
less than 0.2 M, As-sulfide was never formed;
instead porous GaAs was formed; in agreement
with published work [21 – 24]. It is thus clear
that high concentrations of H2SO4 were needed
to obtain a passivated As-sulphide layer;
possibly due to stripping surface oxides from
GaAs prior to depositing the As-sulfide layer.
Figure 3: Scanning electron micrograph of
GaAs after illumination by 25 mW cm-2in a
mixture of 0.8 M TU and 0.3 M H2SO4 after100
scans.
10.00
I x 103, A cm-2
8.00
6.00
4.00
Sulfide
oxidation
2.00
0.00
-2.00 -1 -0.8 -0.6 -0.4 -0.2 0
0.2 0.4 0.6 0.8
-4.00
-6.00
E, V (Ag/AgCl)
Figure 4: Current – voltage scans in a mixture
of 0.3 M H2SO4 and 0.8 M TU after 100 scans in
the dark (───) and under illumination (…..).
Usually, the anodic photocurrents generated due
to the illumination of n-GaAs reach limiting
values that depend solely on the light intensity;
this is not the case for the As-sulfide coated
GaAs, as the photogenerated current, after being
limited, increased slightly at potentials more
positive than 0.8 V as shown in Figs. 4. This
figure also shows an increase in the dark current
due to the As- sulfide coating. The increase in
the anodic dark and are suggested to be due to
the oxidation of the sulphide film.
From the flat band potential value (Efb = -0.8 V
for the sulphide coated GaAs), and the standard
electrode potential of the S2-/S22- redox couple (0.7 V), the oxidation of sulphide is expected to
proceed in accordance to reaction 1 a and b
under illumination and in the dark, respectively:
[25, 26] :
2S2- + 2h+ → S22-, E0 = -0.7 V (Ag/AgCl)
(1a)
2S2- → S22- + 2e-, E0 = -0.7 V (Ag/AgCl)
(1b)
3
1
Oxidation of sulphide in accordance to reaction
1 competes with GaAs dissolution, therefore
sulphide deposition inhibits GaAs corrosion.
corrosion of GaAs was further reduced by about
50% due to As-sulphide deposition.
0.005
0.004
Monolayer / s
Impedance measurements were carried out with
the objective of obtaining the rate of corrosion
of GaAs and As-sulphide, also, determining the
energy bands locations at the semiconductor/
electrolyte interface. The rate of corrosion and
the energy bands positions are obtained from the
charge transfer resistance, Rp, and the flat band
potential, Efb, respectively. These parameters are
determined via the electrochemical analysis of
the impedance Nyquist plots. The fitting and
simulation investigation of these plots shows
that both GaAs and As-sulphide/GaAs
electrolyte interfaces corresponded to the
equivalent
circuit
of
the
type
[R{RQ}{RQ}{RQ}]. An equivalent circuit
similar to the present one has been proposed [27,
28]. The main parameters that were determined
from the electrochemical circuit fit were the
charge transfer resistance, Rp and the space
charge layer capacitance CSC. From the Rp data,
the corrosion current io was calculated. The
number of monolayers depleted due to the
corrosion current io was calculated by assuming
that the number of atoms on GaAs surface is 1x
1015 atom monolayer-1cm-2. Furthermore,
assuming that every surface atom is oxidized to
a trivalent ion, the charge needed to deplete a
monolayer was found to be 1.9 x 10-4 C. From
the impedance results, Rp, and consequently, io,
the corrosion rate in monolayer/s could be
calculated. Fig. 5 shows the relationship
between the corrosion rate and the applied
potential for GaAs and As-sulphide/GaAs under
illumination. This figure clearly shows that Assulphide deposition inhibited the GaAs
dissolution significantly. Relative corrosion
inhibition efficiencies due to As-sulphide
formation was evaluated from the corrosion
rates of the As-sulphide/GaAs electrodes relative
to those of GaAs. It was found that the rates of
corrosion of an illuminated As-sulphide/GaAs
electrode under applied anodic potentials was
about 90% less than those of GaAs. Under
cathodic potentials, the rate of corrosion of both
GaAs and As-sulphide/GaAs was much less than
the anodic corrosion, but the rate of cathodic
0.003
0.002
0.001
0
-1
-0.5
0
0.5
E, V
Figure 5: The relationship between the rate of
corrosion versus the applied potential measured
for GaAs (…..) and sulfide-GaAs (───) under
illumination by 25 mW cm-2.
The positions of the conduction and valence
band edges were determined from the measured
flatband potential Efb values, which were
estimated by the capacitance-voltage relations
(Mott-Schottky analysis). These plots produced
flatband potential values ≈ - 1.2 and - 0.8 V, for
GaAs and As-sulphide/GaAs, respectiveley. The
Efb shifted to more positive potentials with the
As-sulphide deposition. A similar positive shift
was recently reported for GaAs covered with a
monolayer octadecylthiol [29]. The obtained Efb
values, demonstrates convincingly that the
oxidation of GaAs and of As-sulphide/ GaAs
were thermodynamically feasible. The oxidation
of As-sulphide in accordance to reaction 1
competed with the oxidation of GaAs itself, and
thus inhibited its corrosion.
Conclusions.
Acidic TU electrolytes inhibited n-GaAs
corrosion due to the formation of surface Assulphide layer. That layer was continuous and
nonporous if the sulphide deposition was
composed of TU and H2SO4 concentrations >
0.2 M. The oxidation of the surface sulphide
competed with the oxidation of GaAs itself, thus
enhanced the GaAs corrosion inhibition.
4
1
18. K. Adlkofer, M. Tanaka, Langmuir, 17,
4276 (2001).
19. R. Inoue, M. Kitagawa, T. Nishigaki, D.
Morita, K. Ichino, H. Kusano, H.
Kobayashi, Appl. Surf. Sc., 142
341(1999).
20. C.D. Wagner, W.M. Riggs, L.E. Davis,
J.F. Moulder, Handbook of X-ray
Photoelectron Spectroscopy, Physical
Electronics Division, Perkin-Elmer,
MN, 1979.
21. S. Y. Alqaradawi, A. S. Algaber and M.
M. Khader, Thin Solid Films 444, 282
(2003).
22. S.
Langa,
J.
Carstensen,
M.
Christophersen, H. Foll, I. M.
Triginyanu, Appl. Phys. Letters 78,
1074 (2001).
23. P. Schmuki. Fraser, C. M. Vitus, M. J.
Graham and H. S. Isaacs, J.
Electrochem. Soc. 143, 3316 (1996).
24. P. Schmuki, D. J. Lockwood, H. J.
Labbe and J. W. Fraser, Appl. Phys.
Lett. 69 (1996) 1620.
25. A. E. Bolzán, P. L. Schilardi, R.C.V.
Piatti, T. Iwasita, A. Cuesta, C.
Gutiérrez, A. J. Arvia, J. Electroanal.
Chem., 571, 59 (2004).
26. F. M. Al Kharafi, A. Y. Saad, B.G.
Ateya & I.M. Ghayad, Modern Appl.
Sc., 4, 1 (2010).
27. P. Allongue, H. Cachet, J. Electrochem.
Soc., 132, 45 (1985).
28. G. Horowitz, P. Allongue and H.
Cachet, J. Electrochem. Soc. 131 (1984)
2563.
29. K. Adlkofer and M. Tanaka, Langmuir,
17 (2001) 4267.
References:
1. T. Fink, R. M. Osgood, Jr., J .
Electrochem. SOC. 140, L73 (1993).
2. B. J. Tufts, I. L. Abrahams, L. G.
Casagrande, N. S. Lewis, J. Phys.
Chem. 93, 3260 (1989).
3. B. A Parkinson, A. Heller, B. Miller, B.
J . Electrochem. SOC. 126, , 954 (1979).
4. Y. Huang, J. Luo, D. G. Ivey, Thin Solid
Films 496, 724 (2006).
5. I. Belogorokhov, S. A. Gavrilov, I. A.
Belogorokhov, and A. A. Tikhomirov,
Semiconductors, 39, 243 (2005).
Translated from Fizika i Tekhnika
Poluprovodnikov, 39, 258 (2005).
6. H. Taguchi, T. Soga, T. Jimbo, Solar
Energy Materials & Solar Cells, 85, 85
(2005).
7. A. Etcheberry, H. Cachet, R. Cortes and
M. Froment, Surf. Sc. 482, 954 (2001).
8. C. J. Sandroff, R. N. Nottenburg, J. –C.
Bischoff, R. Bhat, Appl. Phys. Lett. 51,
33 (1987).
9. R. N. Nottenburg, C. J. Sandroff, D. A.
Humphrey, T. H. Hollenbeck, and R.
Bhat, Appl. Phys. Lett. 52, 218 (1988).
10. T. Tiedje, P. C. Wong, K. A. R.
Mitchell, W. Eberhardt, F. Zugen, D.
Sondericker, Solid St. Comm., 70, 355
(1989).
11. F. Q. Xu, E. D. Lu, H. B. Pan, C. K.
Xie, P. S. Xu, X. Y. Zhang, Surf. Rev.
& Let. 8, 5 (2001).
12. J. K. Yang, H. H. Park, H. Kim, H. W.
Jang, J. L. Lee, S. Im, Thin Solid Films,
447, 626 (2004).
13. Q. Zhao, G. J. Zhai, R. W. M. Kwok,
Mat. Let., 60, 3084 (2006).
14. P.S. Xu,, F.P. Zhang, E.D. Lu, F.Q. Xu,
H.B. Pan, X.Y. Zhang, Nucl. Instru.and
Methods in Phys. Res. A 467–468, 202
(2001).
15. M. Oshima, T. Scimeca, Y. Watanabe,
H. Oigawa, Y. Nannichi, Jpn. J. Appl.
Phys. 32, 58 (1993).
16. E. A. Miller, G. L. Richmond, J. Phys.
Chem.B, 101, 2669 (1997).
17. S. R. Lunt,
G. N. Ryba, P. G.
Santangelo, N. S. Lewis, J. Appl. Phys.
70, 7449-7467 (1991).
5